5 Ways to Master Double Replacement Reactions
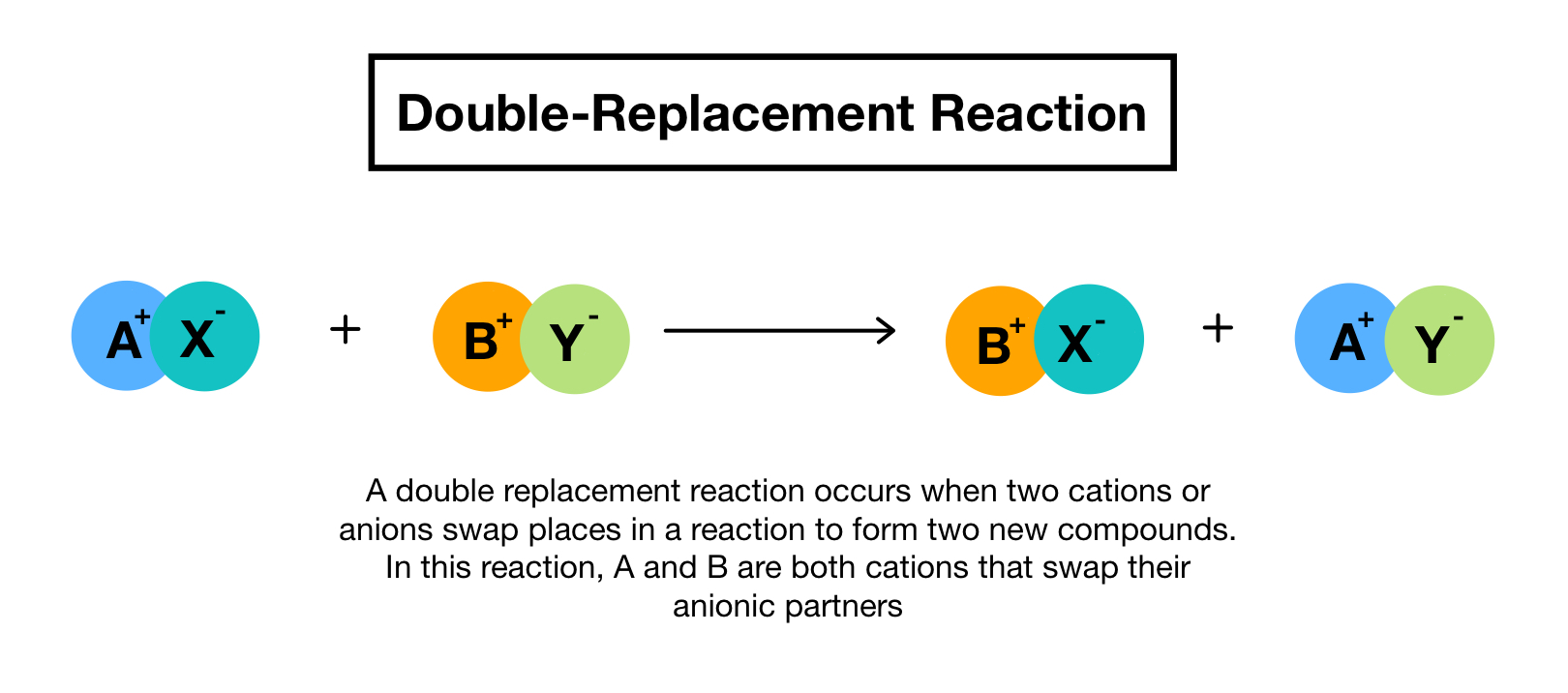
Understanding Double Replacement Reactions
Double replacement reactions, also known as double displacement reactions, are a type of chemical reaction where two compounds react and exchange partners, resulting in the formation of two new compounds. This type of reaction is commonly seen in chemistry and is essential to master for students and professionals alike. In this article, we will explore five ways to master double replacement reactions and provide tips and tricks to help you become a pro.
Method 1: Learn the Basics
Before diving into the world of double replacement reactions, it’s essential to understand the basics. A double replacement reaction involves two compounds, typically ionic compounds, that exchange partners. The general equation for a double replacement reaction is:
AB + CD → AD + CB
Where A and C are cations, and B and D are anions.
Key Points to Remember:
- Double replacement reactions involve the exchange of partners between two compounds.
- The general equation for a double replacement reaction is AB + CD → AD + CB.
- Ionic compounds are typically involved in double replacement reactions.
Method 2: Practice, Practice, Practice
Practice is key to mastering double replacement reactions. Start by practicing simple reactions, such as:
NaCl + AgNO3 → NaNO3 + AgCl
As you become more comfortable, move on to more complex reactions. Try to identify the reactants and products and predict the outcome of the reaction.
Tips:
- Start with simple reactions and gradually move on to more complex ones.
- Use online resources or practice worksheets to practice double replacement reactions.
- Try to identify the reactants and products and predict the outcome of the reaction.
Method 3: Use Flashcards
Flashcards are an excellent way to memorize key terms and concepts related to double replacement reactions. Create flashcards with the reactants on one side and the products on the other. Quiz yourself by covering the products and trying to recall them.
Tips:
- Create flashcards with key terms and concepts related to double replacement reactions.
- Use flashcards to quiz yourself and reinforce your understanding of double replacement reactions.
Method 4: Watch Video Tutorials
Video tutorials are an excellent way to visualize double replacement reactions and understand the concepts behind them. Websites like Khan Academy, Crash Course, and 3Blue1Brown offer excellent video tutorials on double replacement reactions.
Tips:
- Watch video tutorials to visualize double replacement reactions and understand the concepts behind them.
- Take notes and pause the video to reinforce your understanding of key concepts.
Method 5: Create a Concept Map
A concept map is a visual representation of concepts and relationships between them. Create a concept map to illustrate the relationships between double replacement reactions, ionic compounds, and chemical reactions.
Tips:
- Create a concept map to illustrate the relationships between double replacement reactions, ionic compounds, and chemical reactions.
- Use different colors and symbols to represent different concepts and relationships.
📝 Note: Creating a concept map can help you visualize the relationships between different concepts and reinforce your understanding of double replacement reactions.
Common Double Replacement Reactions
Here are some common double replacement reactions:

| Reactants | Products |
|---|---|
| NaCl + AgNO3 | NaNO3 + AgCl |
| CaCO3 + HCl | CaCl2 + H2O + CO2 |
| ZnS + CuSO4 | ZnSO4 + CuS |
Tips:
- Memorize common double replacement reactions to help you recognize patterns and relationships.
- Use the table above to practice identifying reactants and products.
In conclusion, mastering double replacement reactions requires practice, patience, and persistence. By following the five methods outlined above, you can become a pro at double replacement reactions and improve your understanding of chemistry.
What is a double replacement reaction?
+A double replacement reaction is a type of chemical reaction where two compounds react and exchange partners, resulting in the formation of two new compounds.
What is the general equation for a double replacement reaction?
+The general equation for a double replacement reaction is AB + CD → AD + CB.
How can I practice double replacement reactions?
+You can practice double replacement reactions by using online resources, practice worksheets, or creating flashcards.
Related Terms:
- Double replacement Reaction Worksheet answers
- Chemistry replacement reaction Worksheet
- Double replacement reaction quiz
- Balancing double replacement reactions
- Single displacement problems



