Hydrates Composition Worksheet for Chemistry Success
Mastering Hydrates Composition: A Key to Chemistry Success
Hydrates are a fundamental concept in chemistry, and understanding their composition is crucial for achieving success in the field. In this post, we will delve into the world of hydrates, exploring what they are, how they are formed, and how to determine their composition.
What are Hydrates?
Hydrates are compounds that contain water molecules within their crystal structure. These water molecules can be present in various forms, such as water of crystallization, water of hydration, or water of composition. Hydrates can be found in nature, but they can also be synthesized in a laboratory.
Types of Hydrates
There are several types of hydrates, including:
- Monohydrates: These hydrates contain one water molecule for every formula unit of the compound.
- Dihydrates: These hydrates contain two water molecules for every formula unit of the compound.
- Trihydrates: These hydrates contain three water molecules for every formula unit of the compound.
- Polyhydrates: These hydrates contain multiple water molecules for every formula unit of the compound.
How are Hydrates Formed?
Hydrates are formed through various methods, including:
- Crystallization: Hydrates can be formed through the crystallization of a compound from a solution. Water molecules are incorporated into the crystal structure during the crystallization process.
- Chemical Reaction: Hydrates can be formed through chemical reactions, such as the reaction of a metal ion with water.
- Absorption: Hydrates can be formed through the absorption of water vapor by a compound.
Determining Hydrate Composition
Determining the composition of a hydrate is crucial for understanding its properties and behavior. Here are some methods for determining hydrate composition:
- Gravimetric Analysis: This method involves measuring the mass of a hydrate before and after heating it to remove the water molecules. The difference in mass represents the amount of water present in the hydrate.
- Titration: This method involves reacting a hydrate with a strong acid or base to release the water molecules. The amount of acid or base required to react with the hydrate is directly proportional to the amount of water present.
- Spectroscopy: This method involves analyzing the hydrate using spectroscopic techniques, such as infrared spectroscopy or nuclear magnetic resonance spectroscopy. These techniques can provide information on the molecular structure of the hydrate, including the presence of water molecules.

| Hydrate | Composition |
|---|---|
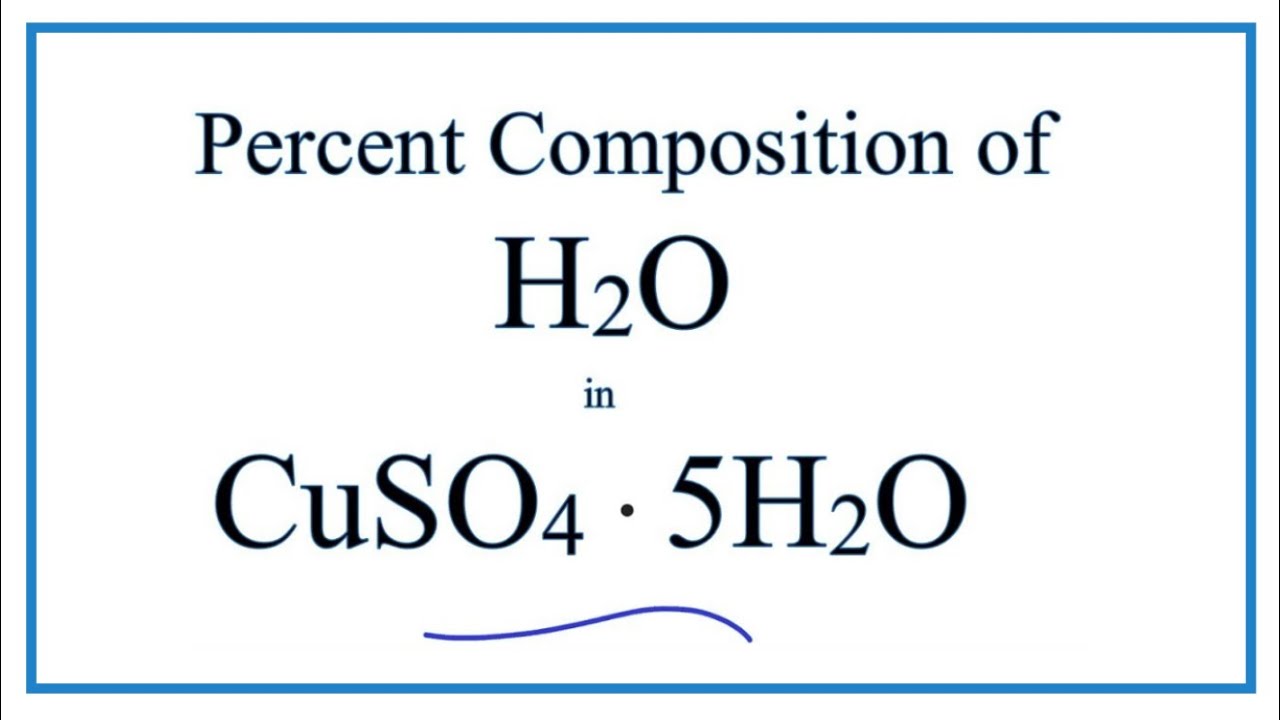
| Copper(II) sulfate pentahydrate | CuSO4·5H2O |
| Barium chloride dihydrate | BaCl2·2H2O |
| Calcium chloride hexahydrate | CaCl2·6H2O |
🔍 Note: When determining the composition of a hydrate, it is essential to consider the possible presence of other solvents or impurities that may affect the results.
Importance of Hydrates in Chemistry
Hydrates play a crucial role in various chemical processes and applications, including:
- Catalysis: Hydrates can act as catalysts in chemical reactions, facilitating the conversion of reactants into products.
- Pharmaceuticals: Hydrates are used as excipients in pharmaceutical formulations, helping to stabilize and control the release of active ingredients.
- Materials Science: Hydrates are used in the development of advanced materials, such as ceramics and composites, due to their unique properties and versatility.
Conclusion
In conclusion, understanding the composition of hydrates is essential for achieving success in chemistry. By mastering the concepts and techniques outlined in this post, chemists can unlock the secrets of hydrates and harness their potential in various fields. Whether you are a student, researcher, or industry professional, a deep understanding of hydrates will serve you well in your pursuit of chemical knowledge and innovation.
What is the difference between a monohydrate and a dihydrate?
+A monohydrate contains one water molecule for every formula unit of the compound, while a dihydrate contains two water molecules for every formula unit of the compound.
How are hydrates formed through crystallization?
+Hydrates are formed through crystallization when a compound is dissolved in water and then allowed to crystallize. The water molecules are incorporated into the crystal structure during the crystallization process.
What is the importance of hydrates in pharmaceuticals?
+Hydrates are used as excipients in pharmaceutical formulations to stabilize and control the release of active ingredients. They can also help to improve the bioavailability and efficacy of drugs.
Related Terms:
- Hydrates Worksheet pdf
- Hydrate's practice problems
- Hydrates Worksheet answer key