Chemistry Significant Digits Worksheet Answers

Understanding Significant Digits in Chemistry
Significant digits, also known as significant figures, are an essential concept in chemistry. They play a crucial role in ensuring the accuracy and reliability of measurements and calculations. In this article, we’ll delve into the world of significant digits, exploring their importance, rules, and applications in chemistry.
Why Significant Digits Matter
Significant digits help chemists convey the precision of their measurements. By using the correct number of significant digits, chemists can avoid miscommunication and ensure that their data is reliable. This is particularly important in chemical reactions, where small errors can have significant consequences.
Rules for Significant Digits
There are several rules to keep in mind when working with significant digits:
- Rule 1: Non-zero digits are always significant. Any digit that is not zero is considered significant.
- Rule 2: Zeros between non-zero digits are significant. Zeros that appear between non-zero digits are also significant.
- Rule 3: Leading zeros are not significant. Zeros that appear before the first non-zero digit are not significant.
- Rule 4: Trailing zeros are significant if the number contains a decimal point. If a number contains a decimal point, trailing zeros are considered significant.
- Rule 5: Trailing zeros are not significant if the number does not contain a decimal point. If a number does not contain a decimal point, trailing zeros are not significant.
Examples of Significant Digits
Let’s consider a few examples to illustrate these rules:
- 456.78: All digits are significant (4, 5, 6, 7, and 8).
- 0.0456: The leading zeros are not significant, but the remaining digits are (4, 5, and 6).
- 4500: The trailing zeros are not significant, so the number has only 2 significant digits (4 and 5).
- 4.500: The trailing zeros are significant because the number contains a decimal point, so the number has 4 significant digits (4, 5, 0, and 0).
Operations with Significant Digits
When performing mathematical operations, the number of significant digits in the result depends on the operation:
- Addition and subtraction: The result should have the same number of decimal places as the number with the fewest decimal places.
- Multiplication and division: The result should have the same number of significant digits as the number with the fewest significant digits.
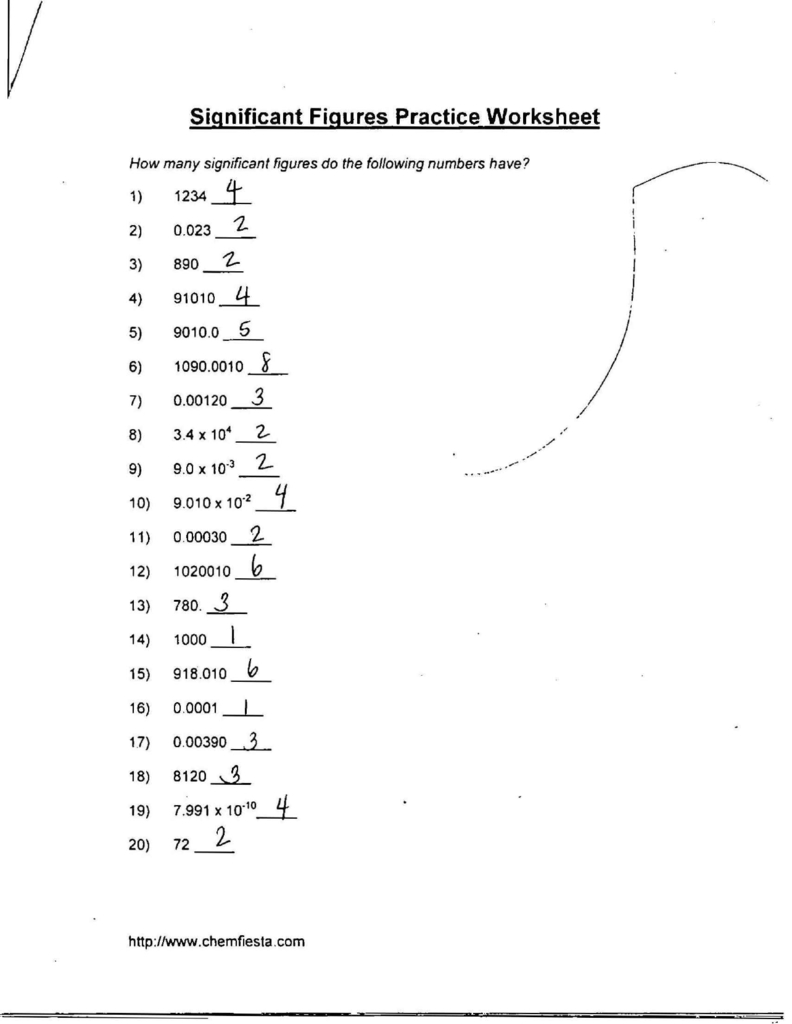
Worksheet Answers
Here are the answers to a chemistry significant digits worksheet:
| Question | Answer |
|---|---|
| What is the number of significant digits in 456.78? | 5 |
| What is the number of significant digits in 0.0456? | 3 |
| What is the number of significant digits in 4500? | 2 |
| What is the result of 456.78 + 0.0456? | 456.83 (rounded to 5 significant digits) |
| What is the result of 456.78 × 0.0456? | 20.8 (rounded to 3 significant digits) |
📝 Note: When performing calculations, always round the result to the correct number of significant digits to ensure accuracy and reliability.
Chemistry Applications
Significant digits play a crucial role in various chemistry applications, including:
- Mole calculations: Significant digits help ensure accurate mole calculations, which are critical in chemical reactions.
- Chemical equations: Significant digits ensure that chemical equations are balanced and accurate.
- Lab measurements: Significant digits help chemists record accurate measurements, which is essential in laboratory experiments.
Conclusion
Significant digits are a fundamental concept in chemistry that ensures the accuracy and reliability of measurements and calculations. By understanding the rules and applications of significant digits, chemists can avoid errors and ensure that their data is trustworthy. Whether you’re a student or a professional chemist, mastering significant digits is essential for success in the field.
What is the purpose of significant digits in chemistry?
+Significant digits help convey the precision of measurements and calculations, ensuring accuracy and reliability in chemistry.
How do I determine the number of significant digits in a number?
+Use the rules for significant digits: non-zero digits are always significant, zeros between non-zero digits are significant, and trailing zeros are significant if the number contains a decimal point.
Why is it important to round results to the correct number of significant digits?
+Rounding results to the correct number of significant digits ensures accuracy and reliability, avoiding errors and miscommunication in chemistry.
Related Terms:
- Significant figures exam
- Scientific notation worksheet