Chemistry Reaction Worksheet for Students
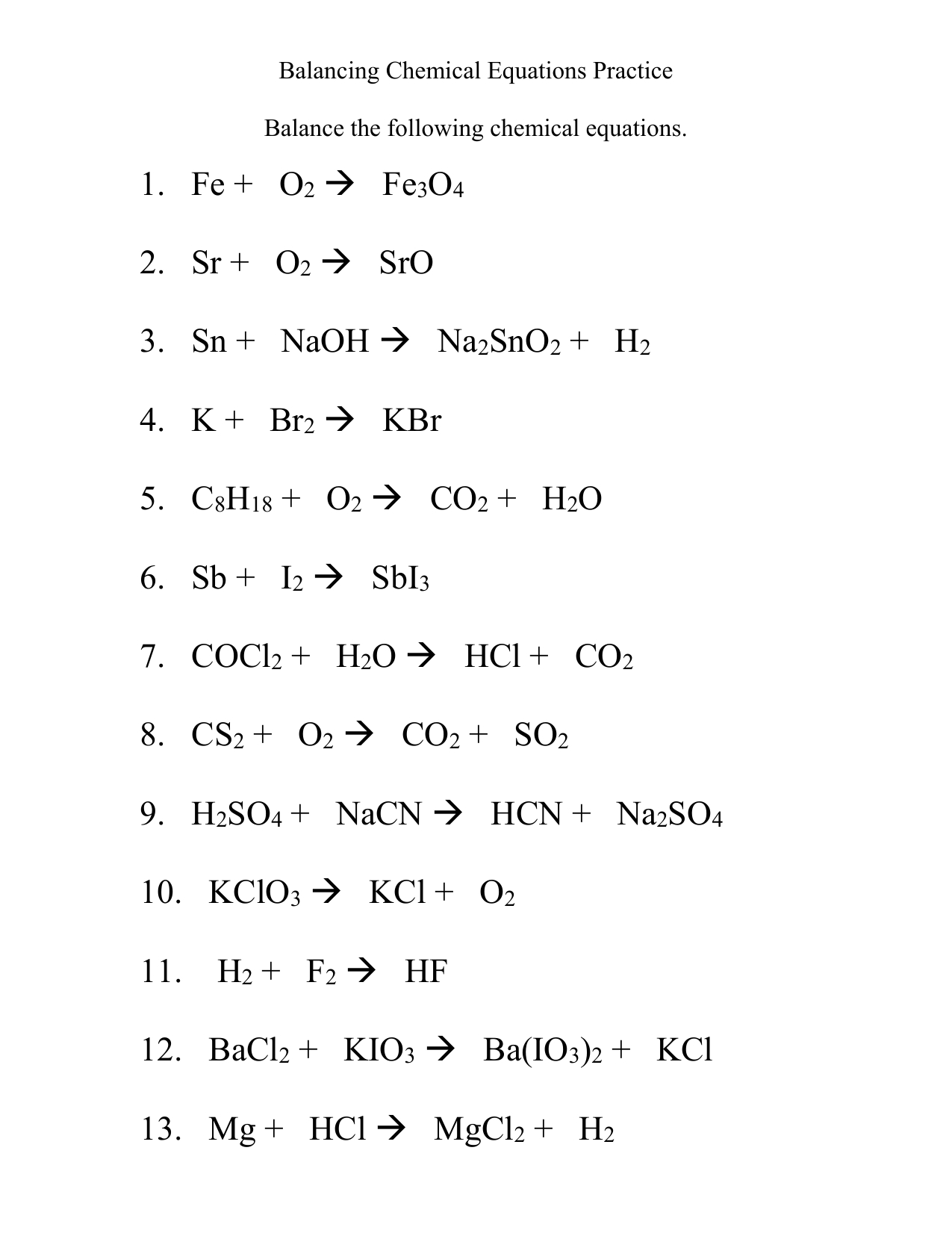
Understanding Chemistry Reactions: A Comprehensive Guide for Students
Chemistry reactions are a crucial part of the study of chemistry, and understanding them is essential for students to grasp the fundamental concepts of the subject. In this worksheet, we will delve into the world of chemistry reactions, exploring the different types, the laws that govern them, and the importance of balancing chemical equations.
What is a Chemistry Reaction?
A chemistry reaction, also known as a chemical reaction, is a process in which one or more substances (reactants) are converted into new substances (products). This process involves the breaking and forming of chemical bonds between atoms, resulting in a change in the chemical composition of the substances involved.
Types of Chemistry Reactions
There are several types of chemistry reactions, including:
- Synthesis reactions: Two or more reactants combine to form a new product.
- Decomposition reactions: A single reactant breaks down into two or more products.
- Replacement reactions: One reactant replaces another reactant in a compound.
- Combustion reactions: A substance reacts with oxygen to produce heat and light.
The Laws of Chemistry Reactions
There are several laws that govern chemistry reactions, including:
- The Law of Conservation of Mass: Matter cannot be created or destroyed in a chemical reaction, only transformed.
- The Law of Definite Proportions: A chemical compound always contains the same proportion of elements by mass.
- The Law of Multiple Proportions: When two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios.
Importance of Balancing Chemical Equations
Balancing chemical equations is crucial in chemistry reactions, as it ensures that the number of atoms of each element is the same on both the reactant and product sides of the equation. This is a fundamental principle in chemistry, as it allows us to predict the outcome of a reaction and understand the chemical changes that occur.
🔍 Note: Balancing chemical equations can be challenging, but it is an essential skill for any chemistry student to master.
Steps to Balance a Chemical Equation
Balancing a chemical equation involves several steps, including:
- Step 1: Write the unbalanced equation, using the correct chemical formulas for each reactant and product.
- Step 2: Count the number of atoms of each element on both the reactant and product sides of the equation.
- Step 3: Add coefficients (numbers in front of the formulas of reactants or products) to balance the equation.
- Step 4: Check the equation to ensure that it is balanced.
Example: Balancing a Chemical Equation
Consider the following unbalanced equation:
Na + Cl2 → NaCl
To balance this equation, we need to add coefficients to ensure that the number of atoms of each element is the same on both sides.
- Step 1: Write the unbalanced equation, using the correct chemical formulas for each reactant and product.
- Step 2: Count the number of atoms of each element on both the reactant and product sides of the equation.

| Element | Reactants | Products |
|---|---|---|
| Na | 1 | 1 |
| Cl | 2 | 1 |
- Step 3: Add coefficients to balance the equation.
2Na + Cl2 → 2NaCl
- Step 4: Check the equation to ensure that it is balanced.
| Element | Reactants | Products |
|---|---|---|
| Na | 2 | 2 |
| Cl | 2 | 2 |
Conclusion
In conclusion, chemistry reactions are a fundamental part of the study of chemistry, and understanding them is essential for students to grasp the fundamental concepts of the subject. By understanding the different types of chemistry reactions, the laws that govern them, and the importance of balancing chemical equations, students can develop a deeper understanding of the subject and apply it to real-world problems.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction involves the combination of two or more reactants to form a new product, while a decomposition reaction involves the breaking down of a single reactant into two or more products.
Why is balancing chemical equations important?
+Balancing chemical equations is important because it ensures that the number of atoms of each element is the same on both the reactant and product sides of the equation, allowing us to predict the outcome of a reaction and understand the chemical changes that occur.
What is the Law of Conservation of Mass?
+The Law of Conservation of Mass states that matter cannot be created or destroyed in a chemical reaction, only transformed.