5 Essential Gas Laws for Chemistry Mastery
Understanding the Building Blocks of Chemistry: Essential Gas Laws
Chemistry, the study of the composition, properties, and reactions of matter, is a vast and intricate field that underlies many aspects of our daily lives. Among its foundational principles, the gas laws stand out as crucial for understanding the behavior of gases and their interactions with other substances. These laws, each contributed by different scientists over time, provide a comprehensive framework for predicting the physical properties and behavior of gases under varying conditions.
The Five Essential Gas Laws
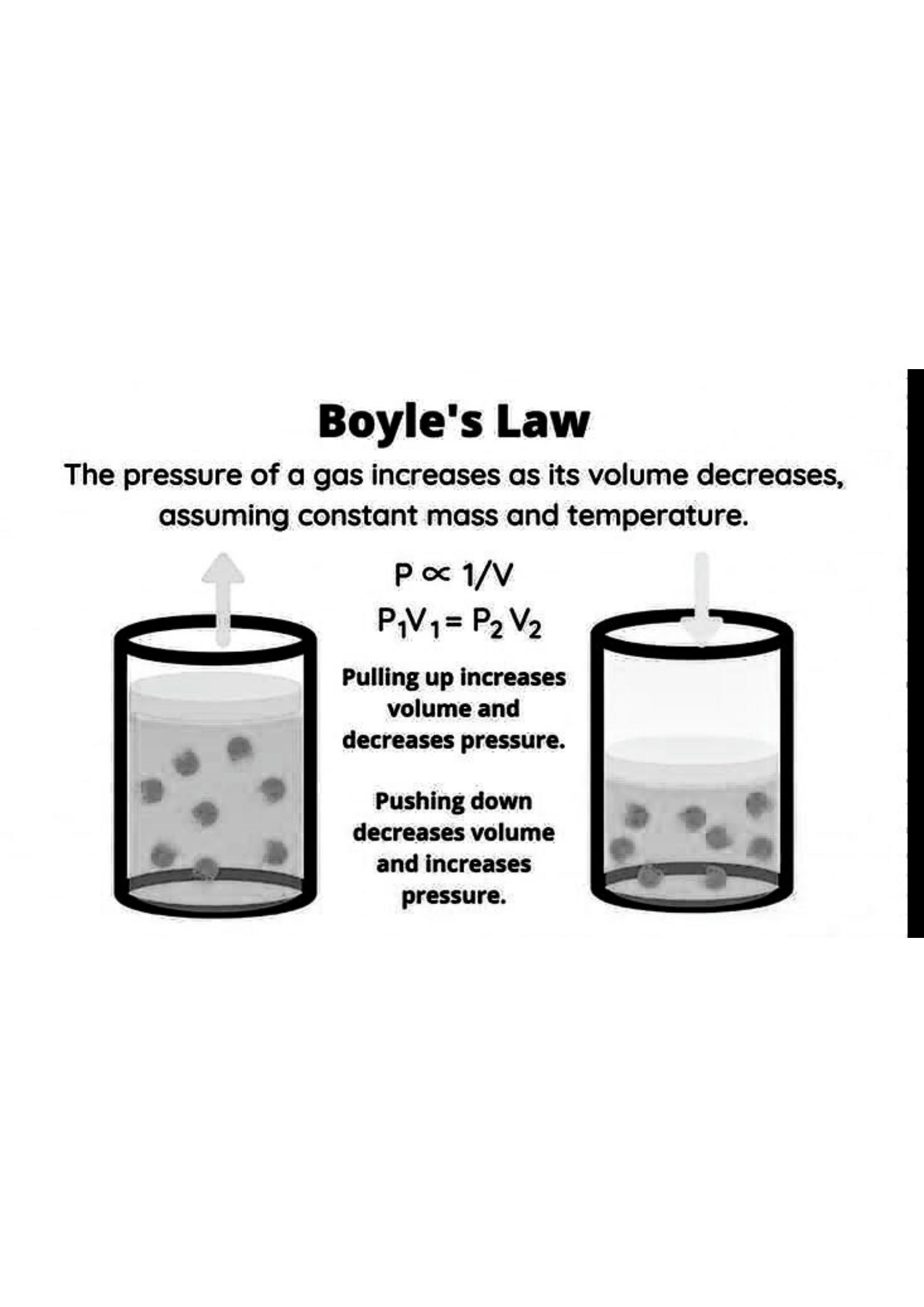
1. Boyle’s Law (1662)
Named after Robert Boyle, this law describes the inverse relationship between the pressure and volume of a gas, provided that the temperature and the amount of gas remain constant. Mathematically, this is represented as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Key Points:
- Inverse Relationship: As the pressure of a gas increases, its volume decreases, assuming a constant temperature. - Constant Conditions: Temperature and the quantity of gas must remain unchanged.2. Charles’s Law (1787)
Charles’s Law, formulated by Jacques Charles, relates the volume of a gas to its temperature, given that the pressure and the amount of gas are kept constant. It states that the volume of a gas is directly proportional to the temperature (in Kelvin), expressed as V1/T1 = V2/T2.
Key Points:
- Direct Proportionality: The volume of a gas increases as its temperature rises, assuming constant pressure. - Temperature in Kelvin: Charles’s Law uses the Kelvin scale for temperature measurements.3. Avogadro’s Law (1811)
Amedeo Avogadro proposed that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. This law allows for the comparison of different gases and is crucial for chemical reactions and stoichiometry.
Key Points:
- Equal Volumes, Equal Molecules: At the same temperature and pressure, equal volumes of different gases have the same number of molecules. - Molar Volume: Avogadro’s Law helps in determining the molar volume of a gas, which is the volume occupied by one mole of the gas.4. Gay-Lussac’s Law (1809)
Joseph Louis Gay-Lussac discovered that, at constant volume, the pressure of a gas is directly proportional to its temperature. This is represented as P1/T1 = P2/T2.
Key Points:
- Direct Proportionality: The pressure of a gas increases as its temperature rises, assuming constant volume. - Constant Volume: This law applies when the volume of the gas is not changed.5. The Ideal Gas Law (1834)
Emile Clapeyron combined the previous laws into a single equation, PV = nRT, known as the Ideal Gas Law, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
Key Points:
- Comprehensive Law: This law accounts for the relationships between pressure, volume, temperature, and the amount of gas. - Assumptions: It assumes ideal behavior of gases, meaning the gas molecules have no volume and there are no intermolecular forces.Notes
🌟 Note: While these laws provide a fundamental understanding of gas behavior, real gases deviate from these laws under extreme conditions.
🌐 Note: The gas laws are foundational in chemistry and physics, with applications in various fields, including engineering, environmental science, and materials science.
Understanding these five gas laws is essential for mastering chemistry, as they form the basis of numerous applications and further studies in chemistry and related sciences.
With a grasp of Boyle’s, Charles’s, Avogadro’s, Gay-Lussac’s Laws, and the Ideal Gas Law, individuals can better comprehend the complex behaviors of gases, their interactions, and the underlying principles that govern their physical properties. These laws are not just historical milestones but active tools in scientific inquiry and problem-solving, bridging the gap between theoretical knowledge and practical applications.
By integrating these fundamental principles into their learning and application, students and practitioners of chemistry can elevate their comprehension and skills, leading to a deeper appreciation of the intricacies of the chemical world and its vast, unexplored territories.
What is the most significant contribution of the gas laws in chemistry?
+The gas laws provide a comprehensive framework for understanding the behavior of gases, allowing for the prediction of physical properties and behavior under various conditions.
How do the gas laws relate to real-world applications?
+The gas laws have applications in engineering, environmental science, materials science, and more, providing insights into gas behavior that are crucial for technological advancements and problem-solving.
What are the limitations of the gas laws?
+The gas laws assume ideal gas behavior, which real gases may deviate from under extreme conditions of temperature and pressure, limiting their application to those specific conditions.