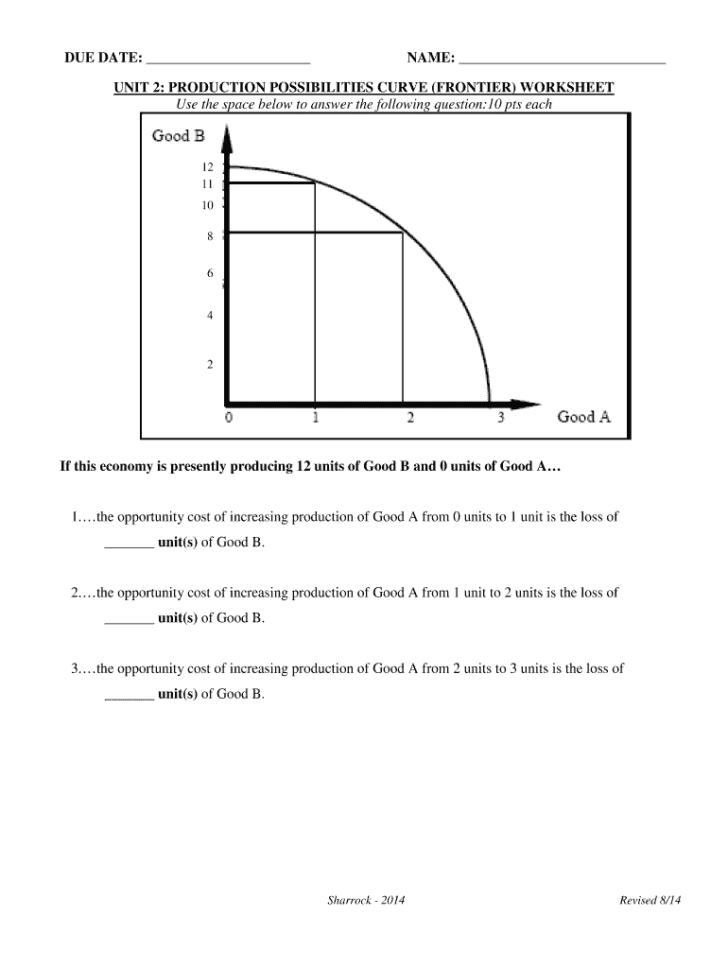
7 Ways to Master Atom Counting in Chemistry

Understanding the Basics of Atom Counting
In chemistry, mastering atom counting is an essential skill that can make a significant difference in understanding chemical reactions, formulas, and equations. Atom counting involves determining the number of atoms of each element present in a molecule or compound. It may seem like a straightforward task, but it can be challenging, especially when dealing with complex molecules or reactions. In this article, we will discuss seven ways to master atom counting in chemistry.
1. Familiarize Yourself with the Periodic Table
The periodic table is a chemist’s best friend. It provides a wealth of information about the elements, including their symbols, atomic numbers, and electron configurations. To master atom counting, you need to be familiar with the periodic table and know how to use it to identify the elements present in a molecule or compound.
Important Elements to Remember:
- Hydrogen (H)
- Oxygen (O)
- Nitrogen (N)
- Carbon ©
- Chlorine (Cl)
🔍 Note: The periodic table is not just a reference tool, but also a guide to understanding the relationships between elements and their properties.
2. Learn to Read Chemical Formulas
Chemical formulas are a shorthand way of representing the composition of molecules and compounds. To master atom counting, you need to learn how to read chemical formulas and understand the relationships between the elements present.
Common Types of Chemical Formulas:
- Molecular formulas (e.g., H2O)
- Empirical formulas (e.g., CH2)
- Structural formulas (e.g., H - O - H)
📝 Note: Pay attention to the subscripts and coefficients in chemical formulas, as they indicate the number of atoms of each element present.
3. Understand the Rules of Nomenclature
Nomenclature is the set of rules used to name compounds. Understanding the rules of nomenclature can help you master atom counting by providing a systematic way of identifying the elements present in a compound.
Key Nomenclature Rules:
- Prefixes indicate the number of atoms of each element (e.g., mono-, di-, tri-)
- Suffixes indicate the type of compound (e.g., -ane, -ene, -ine)
📚 Note: Familiarize yourself with the IUPAC nomenclature rules, which provide a standardized way of naming compounds.
4. Practice Counting Atoms in Simple Molecules
Practice makes perfect, and counting atoms in simple molecules is an excellent way to start. Begin with simple molecules like water (H2O), carbon dioxide (CO2), and methane (CH4).
Example:
- Water (H2O) contains 2 hydrogen atoms and 1 oxygen atom.
👍 Note: Start with simple molecules and gradually move on to more complex ones.
5. Learn to Balance Chemical Equations
Balancing chemical equations is an essential skill in chemistry, and it involves counting atoms. By learning to balance chemical equations, you can master atom counting and develop a deeper understanding of chemical reactions.
Example:
- 2H2 + O2 → 2H2O
📝 Note: Pay attention to the coefficients and subscripts in balanced chemical equations, as they indicate the number of atoms of each element present.
6. Use Online Tools and Resources
There are many online tools and resources available to help you master atom counting. These include interactive periodic tables, molecule builders, and online quizzes.
Recommended Online Resources:
- Periodic Table of Elements (PTABLE)
- Molecule Builder (PHET Interactive Simulations)
- Chemistry Quiz (Khan Academy)
📊 Note: Take advantage of online resources to practice counting atoms and reinforce your understanding of chemical concepts.
7. Review and Practice Regularly
Finally, review and practice regularly to master atom counting. Review the periodic table, chemical formulas, and nomenclature rules regularly, and practice counting atoms in simple and complex molecules.
Example:
- Review the periodic table for 10 minutes each day
- Practice counting atoms in 5 simple molecules each week
📚 Note: Consistency is key when it comes to mastering atom counting. Review and practice regularly to reinforce your understanding of chemical concepts.
Mastering atom counting takes time and practice, but with these seven tips, you can develop a strong foundation in chemistry and improve your understanding of chemical reactions, formulas, and equations. Remember to review and practice regularly, and don’t be afraid to ask for help when needed.
What is the importance of mastering atom counting in chemistry?
+
Mastering atom counting is essential in chemistry as it helps in understanding chemical reactions, formulas, and equations. It also enables chemists to predict the properties and behavior of molecules and compounds.
How can I practice counting atoms in simple molecules?
+
You can practice counting atoms in simple molecules by starting with molecules like water (H2O), carbon dioxide (CO2), and methane (CH4). Use online resources or molecule builders to visualize the molecules and count the atoms.
What are some common mistakes to avoid when counting atoms?
+
Common mistakes to avoid when counting atoms include forgetting to count the subscripts, misunderstanding the coefficients in balanced chemical equations, and neglecting to account for the number of atoms in polyatomic ions.