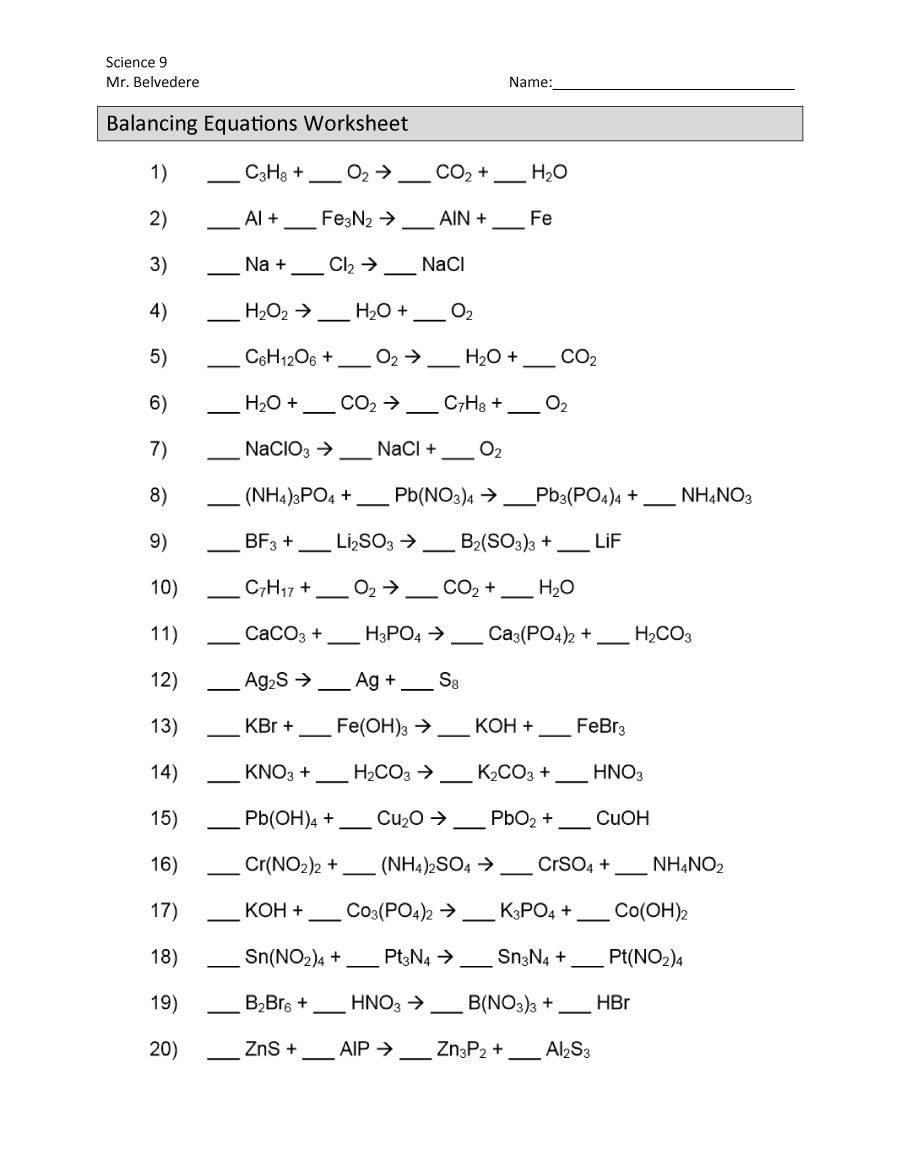
Chemistry Balancing Chemical Equations Worksheet
Understanding the Basics of Balancing Chemical Equations
Balancing chemical equations is a fundamental concept in chemistry that involves ensuring that the number of atoms for each element is the same on both the reactant and product sides of a chemical reaction. This is crucial because it adheres to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. In this worksheet, we will delve into the steps and techniques involved in balancing chemical equations.
The Importance of Balancing Chemical Equations
Balancing chemical equations is important for several reasons:
- It ensures that the chemical reaction is accurate and follows the law of conservation of mass.
- It helps in predicting the amounts of reactants and products involved in a reaction.
- It is essential for calculating the yield of a reaction, which is critical in industrial processes.
Step-by-Step Guide to Balancing Chemical Equations
Balancing chemical equations involves a systematic approach. Here are the steps to follow:
- Write the unbalanced equation: Start by writing the chemical equation with the reactants on the left and the products on the right.
- Count the atoms: Count the number of atoms of each element on both the reactant and product sides.
- Identify the imbalanced elements: Identify the elements that have a different number of atoms on the reactant and product sides.
- Add coefficients: Add coefficients (numbers in front of the formulas of reactants or products) to balance the imbalanced elements. Start with elements that appear only once on each side of the equation.
- Check the balance: After adding coefficients, re-count the atoms to ensure that the equation is balanced.
Example 1: Balancing a Simple Equation
Consider the equation:
[ Na + O_2 \rightarrow Na_2O ]
This equation is not balanced. To balance it:
Count the atoms:
- Reactant side: 1 Na, 2 O
- Product side: 2 Na, 1 O
Identify the imbalanced elements: Na and O.
Add coefficients:
- To balance Na, add a coefficient of 2 in front of Na on the reactant side.
- To balance O, add a coefficient of 2 in front of Na_2O on the product side, but since we’ve already balanced Na, we’ll adjust the coefficient of O_2 instead.
The balanced equation becomes:
[ 4 Na + O_2 \rightarrow 2 Na_2O ]
Notes on Balancing Chemical Equations
- Coefficients vs. Subscripts: Coefficients are numbers placed in front of formulas of reactants or products to balance the equation. Subscripts are small numbers within a chemical formula that indicate the number of atoms of an element in the compound. Never change the subscripts to balance an equation; instead, use coefficients.
- Fractional Coefficients: Sometimes, balancing an equation requires fractional coefficients. However, it’s more conventional to multiply every coefficient by the denominator of the fraction to get whole numbers.
Common Challenges in Balancing Chemical Equations
- Complex Reactions: Some reactions involve multiple reactants and products, making them harder to balance. The key is to take it one step at a time and ensure each element is balanced.
- Oxidation-Reduction (Redox) Reactions: These reactions involve the transfer of electrons. Balancing redox reactions often requires breaking down the reaction into half-reactions and balancing each separately.
🚀 Note: Balancing chemical equations requires patience and attention to detail. With practice, you'll become more proficient in balancing even the most complex equations.
Conclusion
Balancing chemical equations is a fundamental skill in chemistry that ensures chemical reactions adhere to the law of conservation of mass. By following a systematic approach and understanding the principles behind balancing, anyone can master the art of balancing chemical equations. Remember, practice is key, and with time, balancing chemical equations will become second nature.
What is the purpose of balancing chemical equations?
+The purpose of balancing chemical equations is to ensure that the number of atoms for each element is the same on both the reactant and product sides of a chemical reaction, adhering to the law of conservation of mass.
What is the difference between coefficients and subscripts in chemical equations?
+Coefficients are numbers placed in front of formulas of reactants or products to balance the equation, while subscripts are small numbers within a chemical formula that indicate the number of atoms of an element in the compound. Never change the subscripts to balance an equation; instead, use coefficients.
Why is it important to balance chemical equations?
+Balancing chemical equations is important because it ensures that the chemical reaction is accurate and follows the law of conservation of mass. It also helps in predicting the amounts of reactants and products involved in a reaction and is essential for calculating the yield of a reaction, which is critical in industrial processes.
Related Terms:
- Balancing chemical equations Questions
- Balancing chemical equations Activity