Nucleus Particles Worksheet Answer Key and Study Guide
Understanding the Nucleus and Its Particles
The nucleus is the central part of an atom, containing the majority of its mass. It’s composed of protons and neutrons, collectively known as nucleons. In this study guide, we’ll explore the nucleus and its particles, providing you with a comprehensive understanding of the topic.
What is the Nucleus?
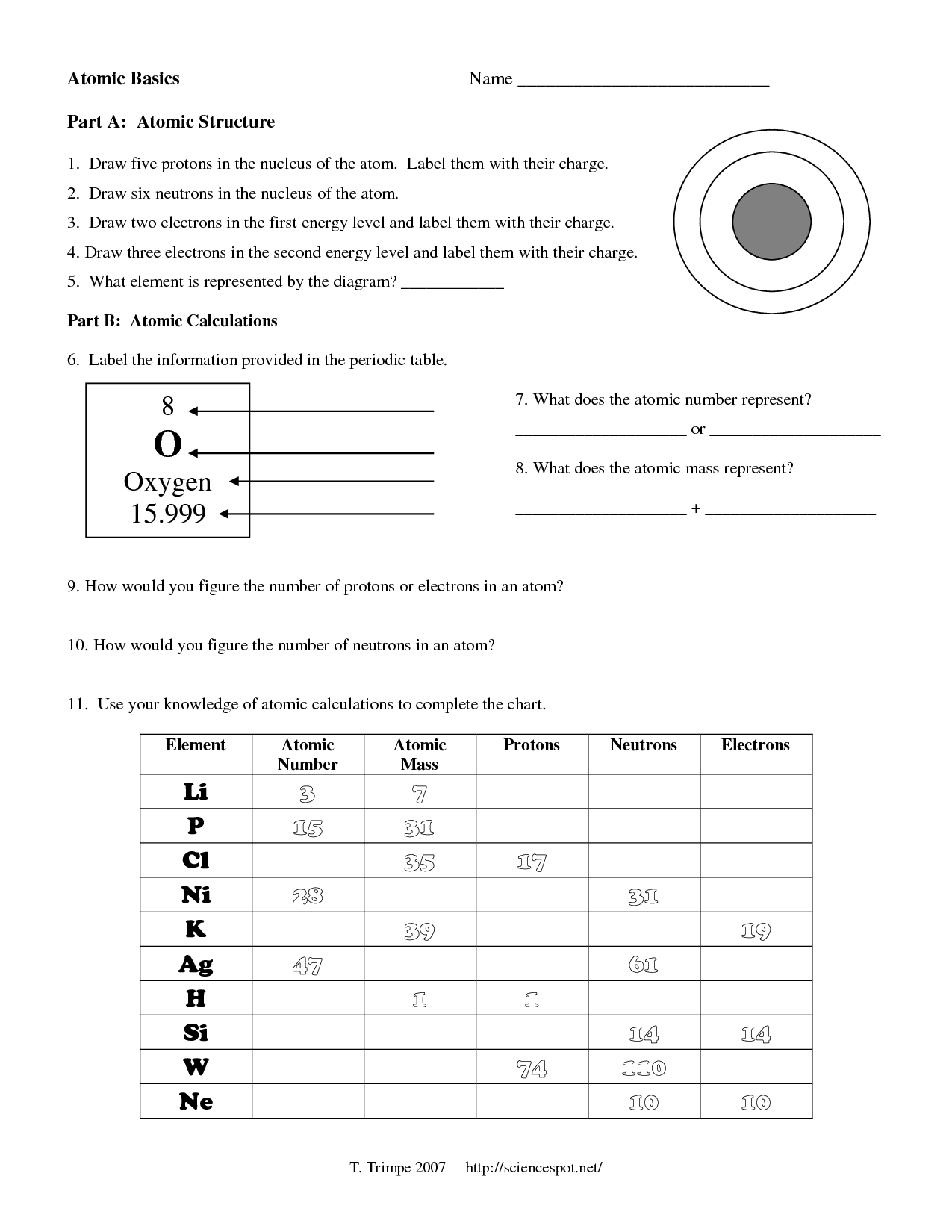
The nucleus is a small, dense region at the center of an atom, containing the protons and neutrons. It’s surrounded by electrons, which orbit the nucleus in energy levels or electron shells. The nucleus is responsible for the overall charge of the atom, with protons contributing to the positive charge and neutrons having no charge.
Protons
Protons are positively charged particles found in the nucleus of an atom. They have a charge of +1 elementary charge and a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom’s nucleus determines the element of an atom, and each element has a unique number of protons in its atoms.
Key Characteristics of Protons:
- Positive charge: +1 elementary charge
- Mass: approximately 1 amu
- Found in the nucleus of an atom
- Determines the element of an atom
Neutrons
Neutrons are particles found in the nucleus of an atom, with no charge and a mass of approximately 1 amu. They contribute to the overall mass of the atom but not to its charge. The number of neutrons in an atom’s nucleus can vary, leading to different isotopes of the same element.
Key Characteristics of Neutrons:
- No charge
- Mass: approximately 1 amu
- Found in the nucleus of an atom
- Contributes to the overall mass of the atom
Nucleus Particles Worksheet Answer Key
Complete the following worksheet to test your understanding of the nucleus and its particles:
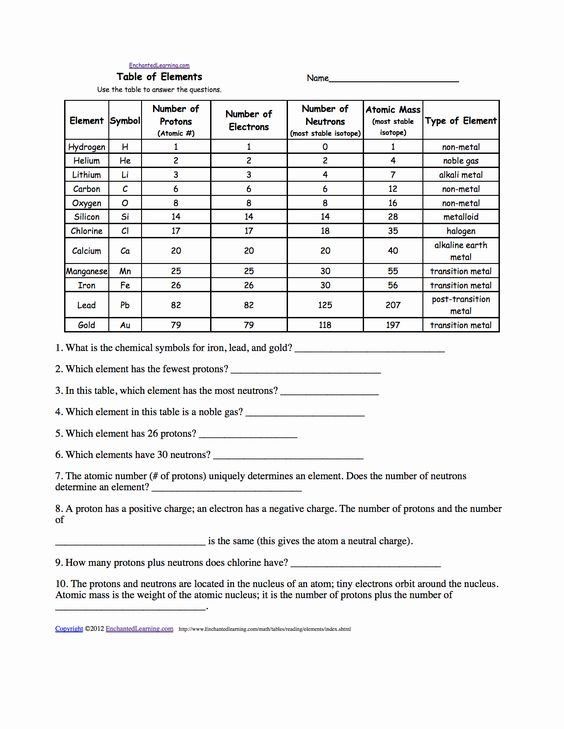
| Question | Answer |
|---|---|
| What is the central part of an atom called? | Nucleus |
| Which particles make up the nucleus? | Protons and neutrons |
| What is the charge of a proton? | +1 elementary charge |
| What determines the element of an atom? | Number of protons in the nucleus |
| What is the mass of a neutron? | Approximately 1 amu |
📝 Note: This worksheet is designed to test your understanding of the nucleus and its particles. Be sure to review the material before attempting to complete the worksheet.
Study Guide Questions
Answer the following questions to reinforce your understanding of the nucleus and its particles:
- What is the main difference between protons and neutrons?
- How do protons and neutrons contribute to the overall mass of an atom?
- What determines the charge of an atom?
- How do electrons relate to the nucleus of an atom?
Answers:
- The main difference between protons and neutrons is their charge. Protons have a positive charge, while neutrons have no charge.
- Protons and neutrons contribute to the overall mass of an atom, with protons having a mass of approximately 1 amu and neutrons having a mass of approximately 1 amu.
- The charge of an atom is determined by the number of protons in its nucleus.
- Electrons orbit the nucleus of an atom in energy levels or electron shells.
Conclusion
In conclusion, the nucleus is the central part of an atom, composed of protons and neutrons. Understanding the properties and characteristics of these particles is essential for grasping the fundamental principles of chemistry and physics. By completing the worksheet and answering the study guide questions, you’ve demonstrated your knowledge of the nucleus and its particles.
What is the nucleus of an atom?
+The nucleus is the central part of an atom, containing the protons and neutrons.
What is the charge of a proton?
+The charge of a proton is +1 elementary charge.
What determines the element of an atom?
+The number of protons in the nucleus determines the element of an atom.
Related Terms:
- Isotope practice worksheet
- Periodic table practice worksheet