6 Easy Ways to Calculate Heat
As we delve into the world of thermodynamics, understanding how to calculate heat becomes an essential skill. Heat is a fundamental concept in physics, engineering, and chemistry, and being able to quantify it is crucial for various applications. In this article, we will explore six easy ways to calculate heat, making it easier for you to grasp this concept.
What is Heat?
Before we dive into the calculations, let’s briefly define what heat is. Heat is a form of energy that is transferred from one body to another due to a temperature difference. It is a measure of the energy in transit, and it is typically measured in units of joules (J) or calories (cal).
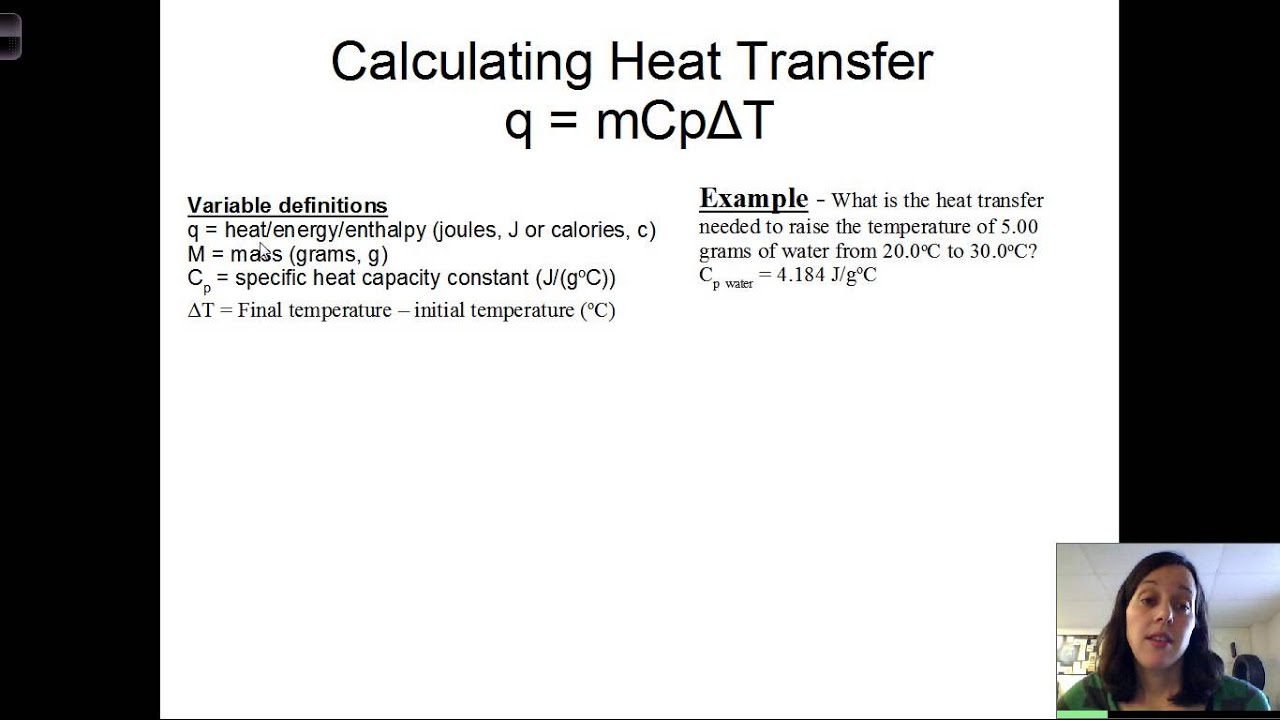
1. Using the Formula Q = mcΔT
One of the most common ways to calculate heat is by using the formula Q = mcΔT, where:
- Q is the amount of heat energy transferred (in J or cal)
- m is the mass of the substance (in kg or g)
- c is the specific heat capacity of the substance (in J/kg°C or cal/g°C)
- ΔT is the change in temperature (in °C)
This formula is widely used to calculate the heat energy transferred between two objects or systems.
2. Calculating Heat Using the Specific Heat Capacity
Another way to calculate heat is by using the specific heat capacity of a substance. The specific heat capacity is the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius.
The formula for specific heat capacity is:
c = Q / (m * ΔT)
Where:
- c is the specific heat capacity (in J/kg°C or cal/g°C)
- Q is the amount of heat energy transferred (in J or cal)
- m is the mass of the substance (in kg or g)
- ΔT is the change in temperature (in °C)
3. Using the Heat Transfer Equation
The heat transfer equation is another way to calculate heat. This equation is used to calculate the heat energy transferred between two objects or systems that are in contact with each other.
The heat transfer equation is:
Q = (T2 - T1) / (1/R1 + 1/R2)
Where:
- Q is the amount of heat energy transferred (in J or cal)
- T1 and T2 are the temperatures of the two objects or systems (in °C)
- R1 and R2 are the thermal resistances of the two objects or systems (in °C/W or °C/cal)
4. Calculating Heat Using the Enthalpy of a System
The enthalpy of a system is the total energy of the system, including the internal energy and the energy associated with the pressure and volume of the system. The enthalpy can be used to calculate the heat energy transferred between two systems.
The formula for enthalpy is:
H = U + pV
Where:
- H is the enthalpy of the system (in J or cal)
- U is the internal energy of the system (in J or cal)
- p is the pressure of the system (in Pa or atm)
- V is the volume of the system (in m³ or L)
5. Using the Calorimeter Equation
A calorimeter is a device used to measure the heat energy transferred between two objects or systems. The calorimeter equation is used to calculate the heat energy transferred between the two objects or systems.
The calorimeter equation is:
Q = m * c * (Tf - Ti)
Where:
- Q is the amount of heat energy transferred (in J or cal)
- m is the mass of the substance (in kg or g)
- c is the specific heat capacity of the substance (in J/kg°C or cal/g°C)
- Tf and Ti are the final and initial temperatures of the substance (in °C)
6. Calculating Heat Using the Thermodynamic Properties of a System
The thermodynamic properties of a system, such as the internal energy, enthalpy, and entropy, can be used to calculate the heat energy transferred between two systems.
The formula for internal energy is:
U = Q - W
Where:
- U is the internal energy of the system (in J or cal)
- Q is the amount of heat energy transferred (in J or cal)
- W is the work done on the system (in J or cal)
| Method | Formula | Description |
|---|---|---|
| Q = mcΔT | Q = mcΔT | Heat energy transferred between two objects or systems |
| Specific Heat Capacity | c = Q / (m \* ΔT) | Specific heat capacity of a substance |
| Heat Transfer Equation | Q = (T2 - T1) / (1/R1 + 1/R2) | Heat energy transferred between two objects or systems in contact |
| Enthalpy of a System | H = U + pV | Total energy of a system, including internal energy and energy associated with pressure and volume |
| Calorimeter Equation | Q = m \* c \* (Tf - Ti) | Heat energy transferred between two objects or systems using a calorimeter |
| Thermodynamic Properties | U = Q - W | Internal energy of a system, using thermodynamic properties |
🔍 Note: The formulas and methods presented in this article are simplified and may not be applicable to all situations. It's essential to consider the specific context and conditions when calculating heat energy.
In summary, calculating heat energy is a crucial aspect of various fields, including physics, engineering, and chemistry. By using the formulas and methods presented in this article, you can easily calculate heat energy transferred between two objects or systems. Remember to consider the specific context and conditions when applying these formulas to ensure accurate results.
What is the difference between heat and temperature?
+Heat and temperature are often confused with each other, but they are distinct concepts. Heat is the energy transferred between two objects or systems due to a temperature difference, while temperature is a measure of the average kinetic energy of the particles in a substance.
What is the unit of measurement for heat energy?
+The unit of measurement for heat energy is typically joules (J) or calories (cal). However, other units such as kilowatt-hours (kWh) or British thermal units (BTU) are also used in specific contexts.
What is the specific heat capacity of water?
+The specific heat capacity of water is approximately 4.184 J/g°C or 1 cal/g°C.
Related Terms:
- Specific heat Calculations Worksheet answers
- Specific heat Worksheet #1
- Calculating specific heat Worksheet
- Heat Practice Problems answer key
- Calculating joules Worksheet
- Heat energy Worksheet pdf