Balancing Nuclear Reactions Made Easy: A Step-by-Step Guide
Understanding the Basics of Nuclear Reactions
Balancing nuclear reactions can seem like a daunting task, but with a clear understanding of the basics, it can be made easy. Nuclear reactions involve changes to the nucleus of an atom, resulting in the transformation of one element into another. These reactions can be either spontaneous or induced, and they can release or absorb energy in the process. In this guide, we will walk you through the steps to balance nuclear reactions, making it easier for you to understand and work with these complex processes.
Step 1: Identify the Reactants and Products
The first step in balancing a nuclear reaction is to identify the reactants and products involved. Reactants are the atoms or nuclei that undergo a transformation, while products are the resulting atoms or nuclei. In a nuclear reaction, the reactants and products are typically represented by their atomic symbols, with the atomic number (number of protons) and mass number (total number of protons and neutrons) included.
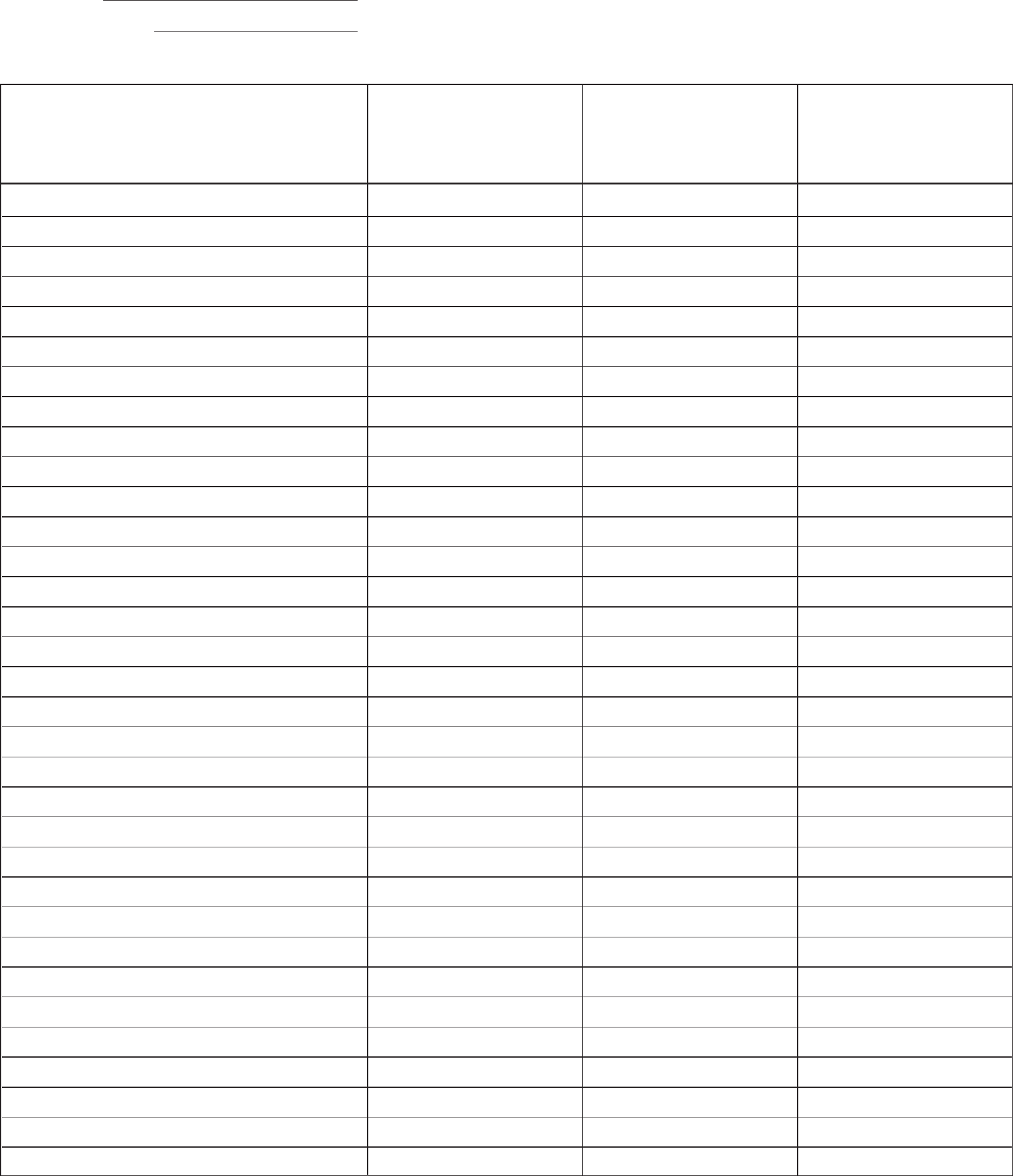
For example, consider the following nuclear reaction:
235U →?
In this reaction, the reactant is uranium-235, and we need to determine the products.
Step 2: Determine the Type of Nuclear Reaction
There are several types of nuclear reactions, including fission, fusion, and radioactive decay. Fission reactions involve the splitting of a heavy nucleus into two or more lighter nuclei, while fusion reactions involve the combination of two or more light nuclei to form a heavier nucleus. Radioactive decay reactions involve the emission of radiation from an unstable nucleus.
In the example above, the reaction involves the splitting of uranium-235, so it is a fission reaction.
Step 3: Balance the Mass Numbers
To balance a nuclear reaction, we need to ensure that the mass numbers of the reactants and products are equal. The mass number is the total number of protons and neutrons in an atom, and it is typically represented by the symbol A.
In our example, the mass number of uranium-235 is 235. To balance the reaction, we need to find products with a total mass number of 235.
Step 4: Balance the Atomic Numbers
In addition to balancing the mass numbers, we also need to balance the atomic numbers of the reactants and products. The atomic number is the number of protons in an atom, and it is typically represented by the symbol Z.
In our example, the atomic number of uranium-235 is 92. To balance the reaction, we need to find products with a total atomic number of 92.
Step 5: Determine the Products
Using the steps above, we can determine the products of the nuclear reaction. In our example, the products are:
235U → 141Ba + 92Kr + 3 1n
In this reaction, uranium-235 splits into barium-141, krypton-92, and three neutrons.
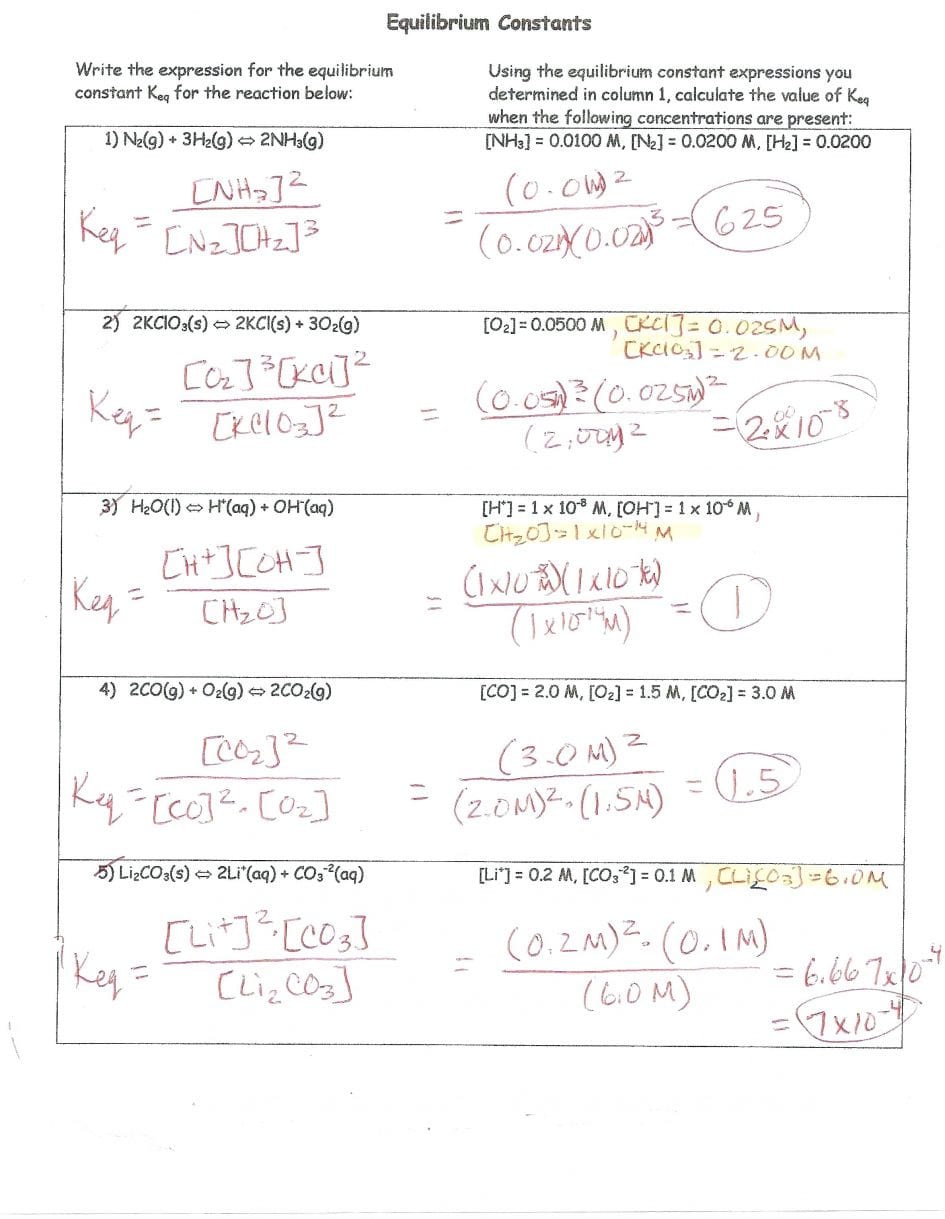
Step 6: Verify the Balance
Finally, we need to verify that the reaction is balanced by checking the mass numbers and atomic numbers of the reactants and products.
| Reactant/Product | Mass Number | Atomic Number |
|---|---|---|
| 235U | 235 | 92 |
| 141Ba | 141 | 56 |
| 92Kr | 92 | 36 |
| 3 1n | 3 | 0 |
| Total | 235 | 92 |
As we can see, the mass numbers and atomic numbers are balanced, verifying that the reaction is correct.
📝 Note: Nuclear reactions can involve complex processes, and it's essential to double-check the balance of the reaction to ensure accuracy.
Conclusion
Balancing nuclear reactions requires a clear understanding of the basics of nuclear physics and the ability to apply the steps outlined above. By following these steps, you can easily balance nuclear reactions and gain a deeper understanding of these complex processes.
What is the difference between fission and fusion reactions?
+Fission reactions involve the splitting of a heavy nucleus into two or more lighter nuclei, while fusion reactions involve the combination of two or more light nuclei to form a heavier nucleus.
What is the purpose of balancing nuclear reactions?
+Balancing nuclear reactions ensures that the mass numbers and atomic numbers of the reactants and products are equal, verifying that the reaction is correct and accurate.
What are some common applications of nuclear reactions?
+Nuclear reactions have various applications, including nuclear power generation, medical treatments, and scientific research.
Related Terms:
- Nuclear equations WORKSHEET pdf
- Balancing nuclear equations worksheet doc
- Balancing nuclear equations Calculator
- Balancing nuclear equations practice
- Nuclear decay equations worksheet pdf