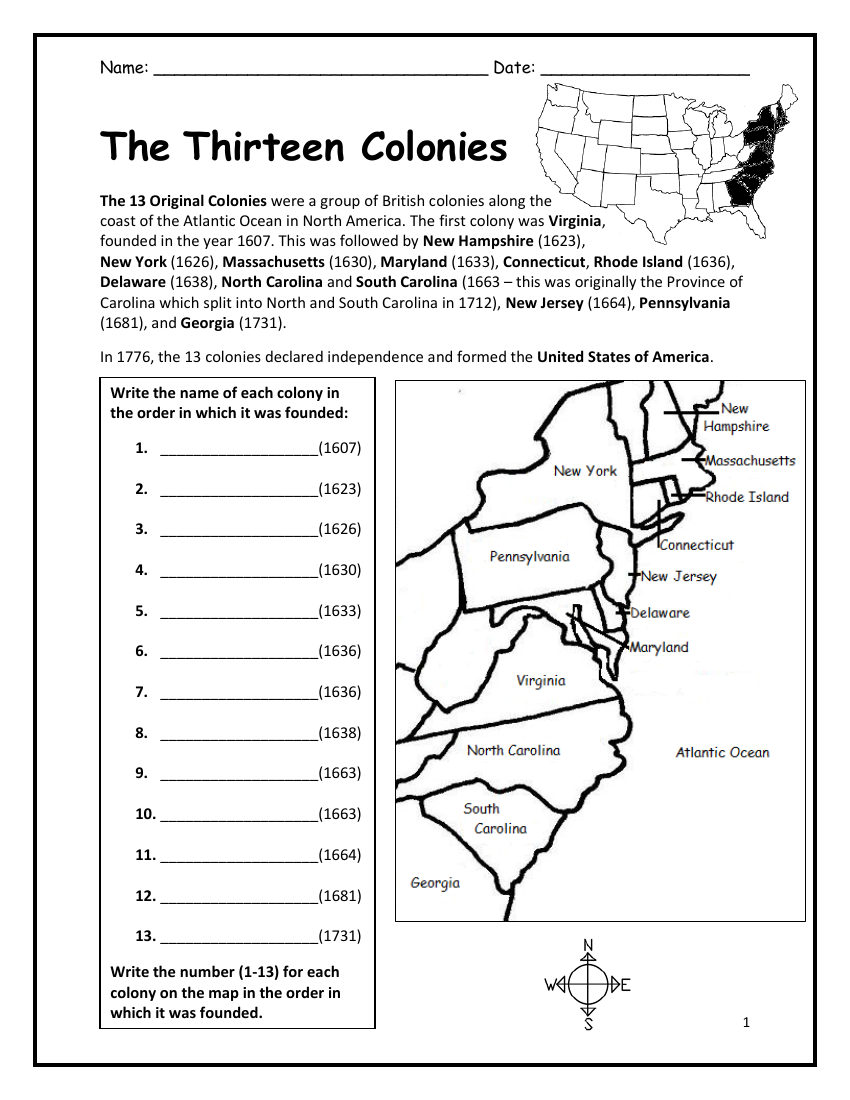
7 Ways to Balance Equations Like a Pro
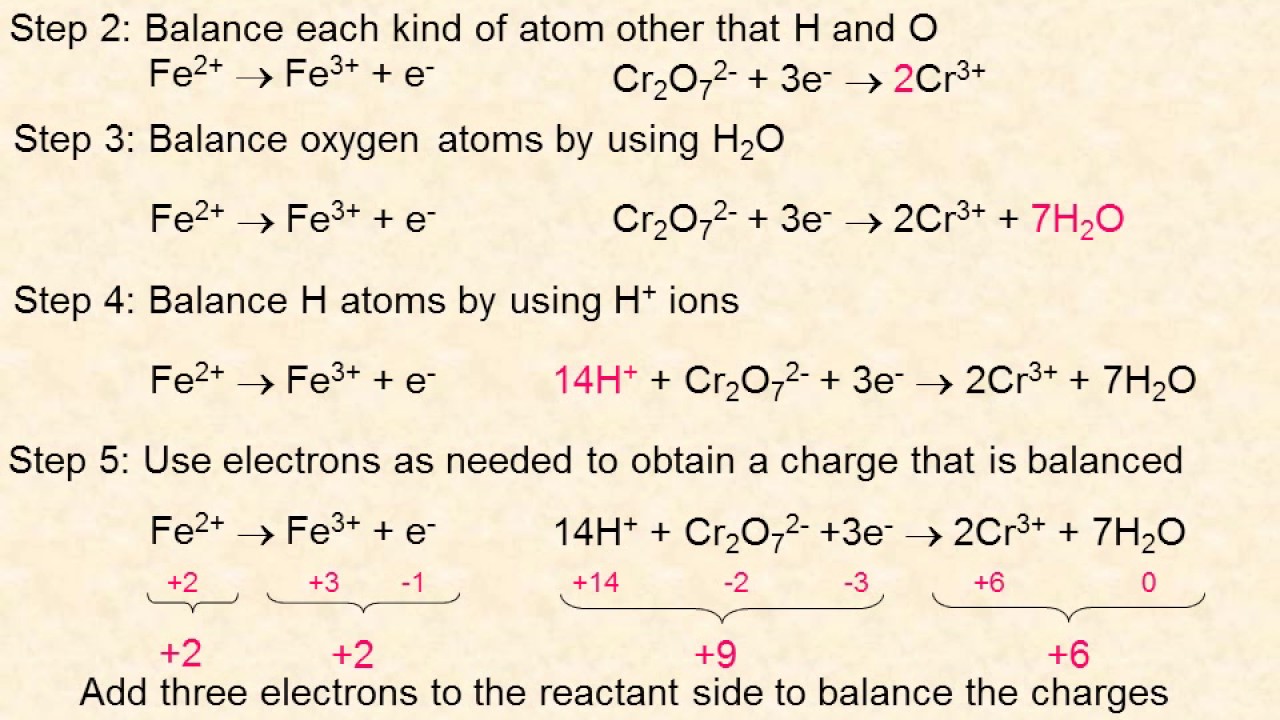
Mastering the Art of Balancing Equations
Balancing chemical equations is a fundamental skill in chemistry that can seem daunting at first, but with practice and the right strategies, it can become second nature. In this article, we will explore seven ways to balance equations like a pro, including tips, tricks, and best practices to help you master this essential skill.
Understanding the Basics of Balancing Equations
Before we dive into the nitty-gritty of balancing equations, it’s essential to understand the basics. A balanced chemical equation is one where the number of atoms of each element is the same on both the reactant and product sides. This is achieved by adding coefficients (numbers in front of the formulas of reactants or products) to the equation.
Key Principles of Balancing Equations
- Atoms cannot be created or destroyed: The number of atoms of each element must be the same on both sides of the equation.
- Coefficients are added, not subtracted: You can only add coefficients to the equation, never subtract them.
- Start with elements that appear only once: Begin by balancing elements that appear only once on each side of the equation.
7 Ways to Balance Equations Like a Pro
1. Use the Step-by-Step Method
This method involves balancing the equation one element at a time, starting with elements that appear only once on each side.
- Step 1: Write down the unbalanced equation.
- Step 2: Identify the elements that appear only once on each side.
- Step 3: Balance the first element by adding a coefficient.
- Step 4: Move on to the next element and repeat steps 2-3 until the equation is balanced.
2. Use the Half-Reaction Method
This method involves splitting the equation into two half-reactions, one for oxidation and one for reduction.
- Step 1: Write down the unbalanced equation.
- Step 2: Identify the elements that are oxidized or reduced.
- Step 3: Write the half-reactions for oxidation and reduction.
- Step 4: Balance the half-reactions by adding coefficients.
- Step 5: Combine the balanced half-reactions to form the final balanced equation.
3. Use the Inspection Method
This method involves inspecting the equation and using your knowledge of chemistry to make educated guesses about the coefficients.
- Step 1: Write down the unbalanced equation.
- Step 2: Inspect the equation and look for patterns or relationships between elements.
- Step 3: Make educated guesses about the coefficients and add them to the equation.
- Step 4: Check the equation to ensure it is balanced.
4. Use the Trial-and-Error Method
This method involves using trial and error to find the correct coefficients.
- Step 1: Write down the unbalanced equation.
- Step 2: Add coefficients to the equation and check if it is balanced.
- Step 3: If the equation is not balanced, adjust the coefficients and try again.
5. Use the Algebraic Method
This method involves using algebra to solve for the coefficients.
- Step 1: Write down the unbalanced equation.
- Step 2: Assign variables to the coefficients.
- Step 3: Write equations based on the number of atoms of each element.
- Step 4: Solve the equations for the coefficients.
6. Use Online Tools and Software
There are many online tools and software programs available that can help you balance equations.
- Step 1: Find a reliable online tool or software program.
- Step 2: Enter the unbalanced equation into the program.
- Step 3: The program will provide the balanced equation.
7. Practice, Practice, Practice
The best way to become proficient at balancing equations is to practice regularly.
- Step 1: Find a source of unbalanced equations (e.g., a textbook or online resource).
- Step 2: Choose an equation to balance.
- Step 3: Try to balance the equation using one of the methods above.
- Step 4: Check your answer and try again if necessary.
📝 Note: The key to mastering balancing equations is practice. Start with simple equations and gradually move on to more complex ones.
Balancing chemical equations is a skill that requires patience, persistence, and practice. By using one or more of the methods outlined above, you can become proficient at balancing equations and improve your overall understanding of chemistry.
Without mastering the art of balancing equations, it is challenging to succeed in chemistry. By following the tips and strategies outlined in this article, you can become a pro at balancing equations and take your chemistry skills to the next level.
What is the most important principle of balancing equations?
+The most important principle of balancing equations is that atoms cannot be created or destroyed, meaning the number of atoms of each element must be the same on both sides of the equation.
What is the half-reaction method?
+The half-reaction method involves splitting the equation into two half-reactions, one for oxidation and one for reduction, and balancing each half-reaction separately.
What is the best way to practice balancing equations?
+The best way to practice balancing equations is to start with simple equations and gradually move on to more complex ones, using a variety of methods and online tools and software programs.