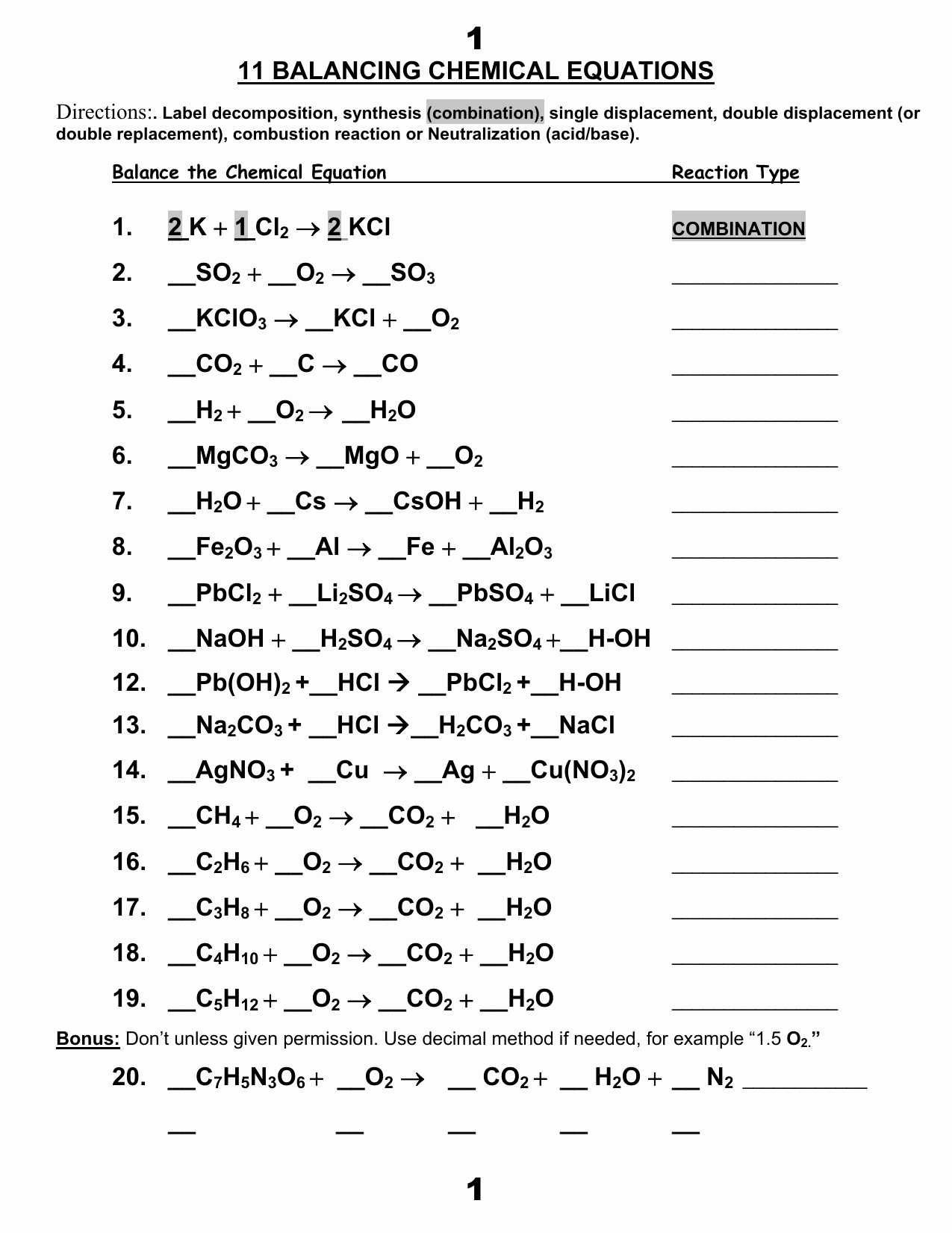
Balancing Equations Worksheet 2 Answer Key Made Easy
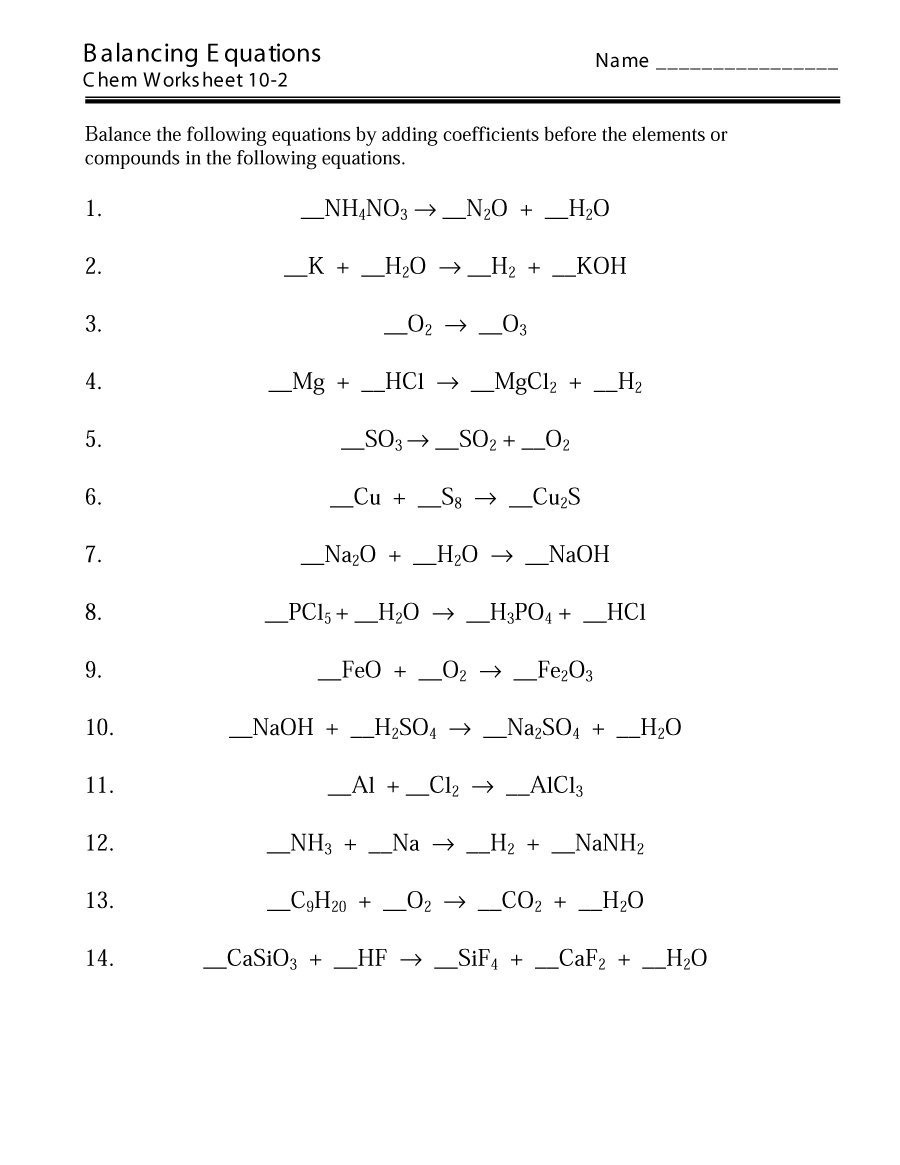
Understanding Balancing Equations: A Comprehensive Guide
Balancing equations is a fundamental concept in chemistry that can seem daunting at first, but with practice and the right approach, it can become a breeze. In this article, we will delve into the world of balancing equations, provide a step-by-step guide on how to balance them, and offer tips and tricks to make the process easier.
What is Balancing Equations?
Balancing equations is the process of ensuring that the number of atoms of each element is the same on both the reactant and product sides of a chemical equation. This is a crucial step in writing accurate chemical equations, as it ensures that the law of conservation of mass is obeyed.
Step-by-Step Guide to Balancing Equations
Balancing equations can be a straightforward process if you follow these simple steps:
- Step 1: Write the unbalanced equation. Start by writing the unbalanced equation with the reactants on the left and the products on the right.
- Step 2: Count the atoms. Count the number of atoms of each element on both the reactant and product sides.
- Step 3: Identify the imbalances. Identify which elements have an unequal number of atoms on both sides.
- Step 4: Balance the elements. Start balancing the elements by adding coefficients (numbers in front of the formulas of reactants or products) to the elements that are imbalanced.
- Step 5: Check your work. Once you think you have balanced the equation, re-count the atoms to ensure that the number of atoms of each element is the same on both sides.
💡 Note: It's essential to balance elements one at a time, starting with the elements that appear most frequently in the equation.
Example: Balancing the Equation for the Combustion of Methane
Let’s use the combustion of methane as an example to illustrate the step-by-step process:
Unbalanced Equation: CH₄ + O₂ → CO₂ + H₂O
Step 1: Count the atoms
| Element | Reactants | Products |
|---|---|---|
| C | 1 | 1 |
| H | 4 | 2 |
| O | 2 | 3 |
Step 2: Identify the imbalances
- Hydrogen (H) has 4 atoms on the reactant side and 2 atoms on the product side.
- Oxygen (O) has 2 atoms on the reactant side and 3 atoms on the product side.
Step 3: Balance the elements
- To balance hydrogen, add a coefficient of 2 in front of H₂O on the product side.
- To balance oxygen, add a coefficient of 2 in front of O₂ on the reactant side.
Balanced Equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Tips and Tricks for Balancing Equations
Here are some additional tips and tricks to help you balance equations with ease:
- Start with the elements that appear most frequently. This will make it easier to balance the equation.
- Use coefficients, not subscripts. Coefficients are numbers in front of the formulas of reactants or products, while subscripts are small numbers that are part of the formula.
- Balance elements one at a time. This will help you avoid confusion and ensure that you don’t introduce new imbalances.
- Check your work. Re-count the atoms to ensure that the number of atoms of each element is the same on both sides.
| Element | Reactants | Products |
|---|---|---|
| C | 1 | 1 |
| H | 4 | 4 |
| O | 4 | 4 |
📝 Note: The table above shows the balanced equation for the combustion of methane, with the number of atoms of each element equal on both sides.
In conclusion, balancing equations is a crucial step in writing accurate chemical equations. By following the step-by-step guide and tips and tricks outlined in this article, you’ll be able to balance equations with ease.
What is the law of conservation of mass?
+The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
What is the difference between a coefficient and a subscript?
+A coefficient is a number in front of the formula of a reactant or product, while a subscript is a small number that is part of the formula.
How do I know if I’ve balanced an equation correctly?
+To ensure that you’ve balanced an equation correctly, re-count the atoms to ensure that the number of atoms of each element is the same on both sides.