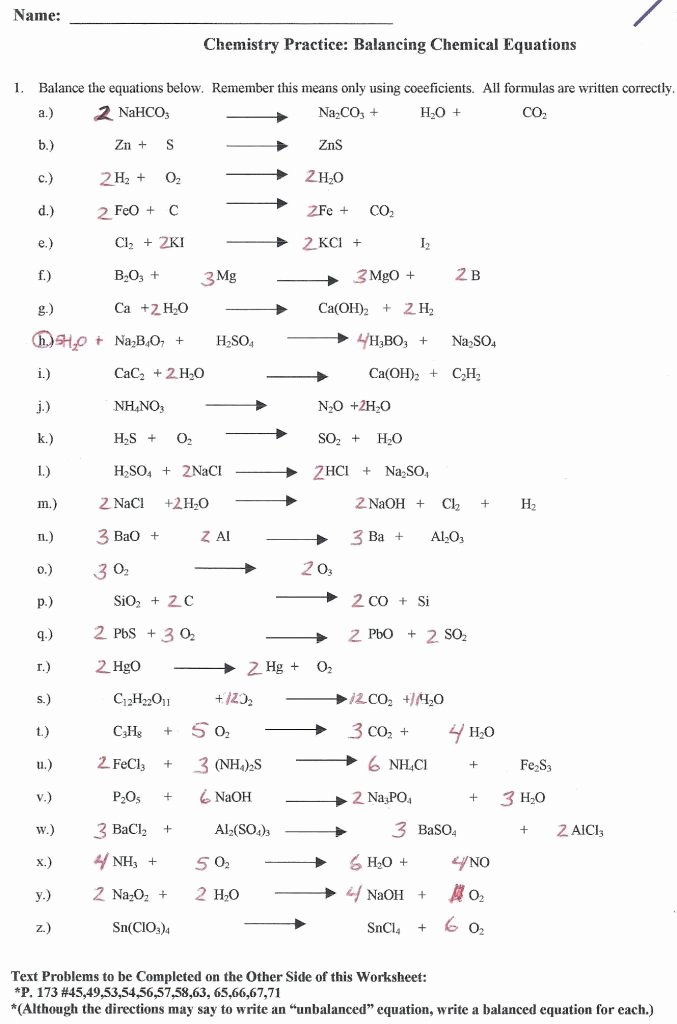
Balancing Chemical Equations Made Easy With Worksheets
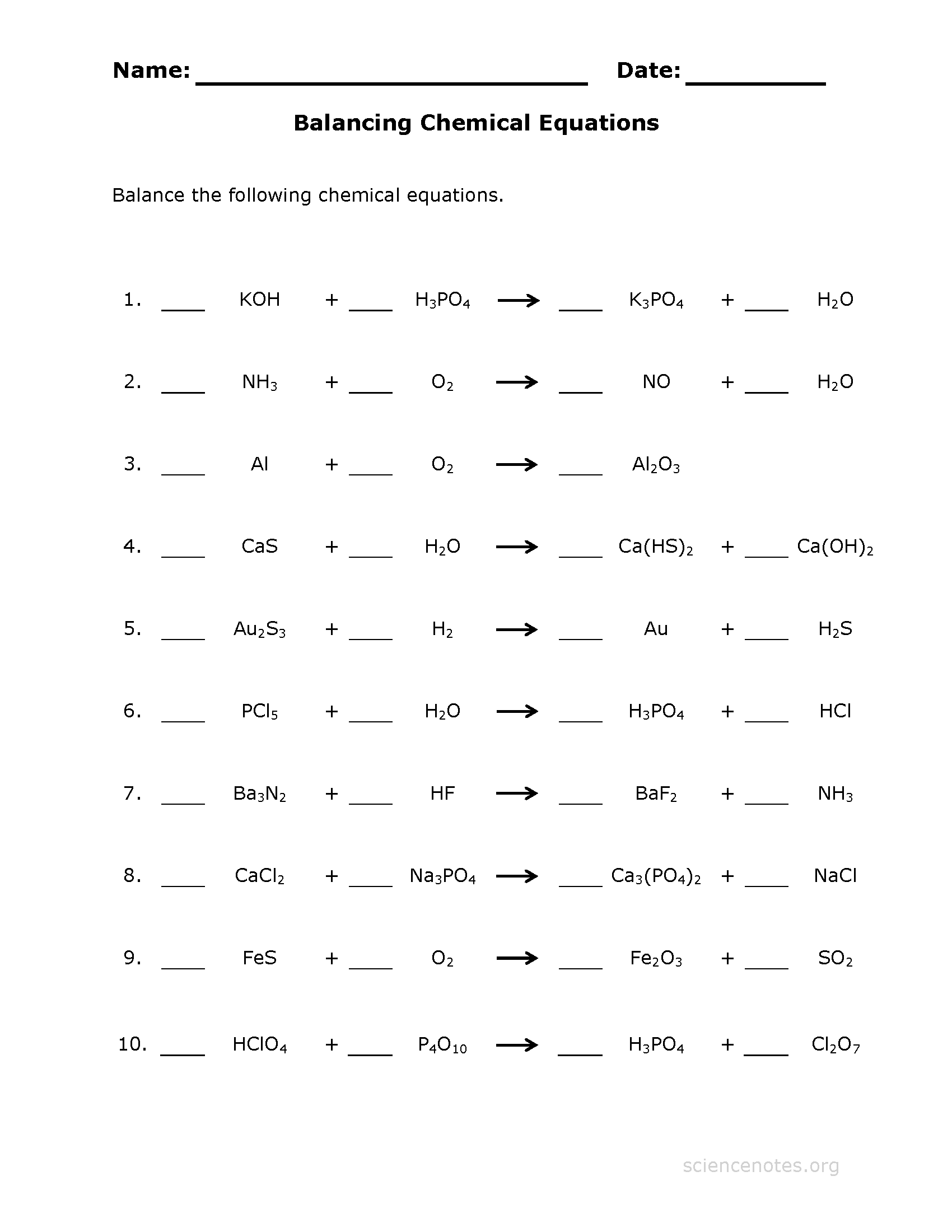
Understanding the Basics of Chemical Equations
Chemical equations are a fundamental concept in chemistry, representing the reactants, products, and reaction conditions of a chemical reaction. A balanced chemical equation is crucial for understanding the stoichiometry of a reaction, which is essential for predicting the amount of reactants and products involved. However, balancing chemical equations can be a daunting task, especially for students new to chemistry. In this post, we will explore how worksheets can make balancing chemical equations easier and more manageable.
What is a Balanced Chemical Equation?
A balanced chemical equation is an equation where the number of atoms of each element is the same on both the reactant and product sides. This is represented by a set of coefficients (numbers in front of the formulas of reactants or products) that multiply the number of atoms of each element. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, which means that the total number of atoms of each element must be conserved.
Why are Worksheets Useful for Balancing Chemical Equations?
Worksheets are an excellent tool for balancing chemical equations because they provide a structured approach to solving the problem. A worksheet typically consists of a table with columns for reactants, products, and coefficients. By using a worksheet, students can systematically balance the equation, element by element, without getting overwhelmed by the complexity of the reaction.
Step-by-Step Guide to Balancing Chemical Equations with Worksheets
Here is a step-by-step guide to balancing chemical equations using worksheets:
- Write the unbalanced equation: Start by writing the reactants and products of the reaction, without worrying about balancing the equation.
- Count the atoms: Count the number of atoms of each element on both the reactant and product sides.
- Identify the imbalance: Identify the elements that have a different number of atoms on the reactant and product sides.
- Add coefficients: Add coefficients in front of the formulas of reactants or products to balance the equation. Start by adding coefficients to the elements that have the greatest imbalance.
- Check the balance: After adding coefficients, re-count the number of atoms of each element to ensure that the equation is balanced.
- Repeat the process: Continue adding coefficients and re-checking the balance until the equation is fully balanced.
📝 Note: It's essential to check the balance of the equation after adding each coefficient to avoid introducing new imbalances.
Example of Balancing a Chemical Equation with a Worksheet
Let’s consider the combustion reaction of methane:
CH₄ + O₂ → CO₂ + H₂O
Using a worksheet, we can balance this equation as follows:
| Reactants | Products | Coefficients |
|---|---|---|
| CH₄ | CO₂ | 1 |
| O₂ | H₂O | 2 |
After counting the atoms, we identify the imbalance:
- Carbon: 1 on the reactant side, 1 on the product side (balanced)
- Hydrogen: 4 on the reactant side, 2 on the product side (imbalanced)
- Oxygen: 2 on the reactant side, 3 on the product side (imbalanced)
We add coefficients to balance the equation:
- Add a coefficient of 2 in front of CH₄ to balance the hydrogen atoms
- Add a coefficient of 2 in front of O₂ to balance the oxygen atoms
The balanced equation becomes:
2CH₄ + 4O₂ → 2CO₂ + 4H₂O
📝 Note: The coefficients can be adjusted further to simplify the equation, but the above equation is already balanced.
Benefits of Using Worksheets for Balancing Chemical Equations
Using worksheets for balancing chemical equations has several benefits:
- Systematic approach: Worksheets provide a step-by-step approach to balancing equations, reducing errors and confusion.
- Improved understanding: By identifying the imbalances and adding coefficients, students gain a deeper understanding of the chemical reaction.
- Reduced complexity: Worksheets break down the complexity of the reaction, making it more manageable for students.
Conclusion
Balancing chemical equations is a crucial skill in chemistry, and worksheets can make this process more manageable and efficient. By following a step-by-step approach and using worksheets, students can balance chemical equations with ease and accuracy. With practice and patience, balancing chemical equations becomes a breeze, and students can focus on more advanced concepts in chemistry.
What is the law of conservation of mass?
+The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, which means that the total number of atoms of each element must be conserved.
Why are coefficients used in balancing chemical equations?
+Coefficients are used to balance the equation by multiplying the number of atoms of each element. This ensures that the number of atoms of each element is the same on both the reactant and product sides.
What are the benefits of using worksheets for balancing chemical equations?
+Worksheets provide a systematic approach to balancing equations, reducing errors and confusion. They also improve understanding and reduce complexity, making it easier for students to balance chemical equations.
Related Terms:
- Balancing chemical equations