5 Ways to Master Balance Equations in Chemistry
Understanding Balance Equations in Chemistry
Balance equations are a fundamental concept in chemistry, and mastering them is crucial for any chemistry student or professional. A balance equation represents a chemical reaction where the number of atoms for each element is the same on both the reactant and product sides. In this blog post, we will explore five ways to master balance equations in chemistry.
Method 1: Counting Atoms
The first step to balancing an equation is to count the number of atoms for each element on both the reactant and product sides. Start by counting the atoms in the reactants and then count the atoms in the products. Make a table or list to help you keep track of the number of atoms for each element.
| Element | Reactants | Products |
|---|---|---|
| Hydrogen (H) | 2 | 2 |
| Oxygen (O) | 1 | 1 |
By counting the atoms, you can identify which elements are not balanced.
Method 2: Balancing Elements One by One
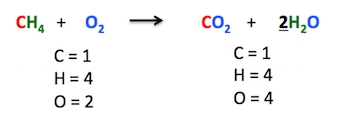
Once you have identified the elements that are not balanced, start balancing them one by one. Begin with the elements that appear only once on each side of the equation. For example, if you have an equation with only one atom of oxygen on the reactant side and two atoms of oxygen on the product side, you can balance the oxygen by adding a coefficient of 2 in front of the reactant.
Example: H₂ + O₂ → H₂O
Unbalanced: H₂ + O₂ → H₂O (oxygen is not balanced)
Balanced: H₂ + 2O₂ → 2H₂O
Method 3: Using Coefficients
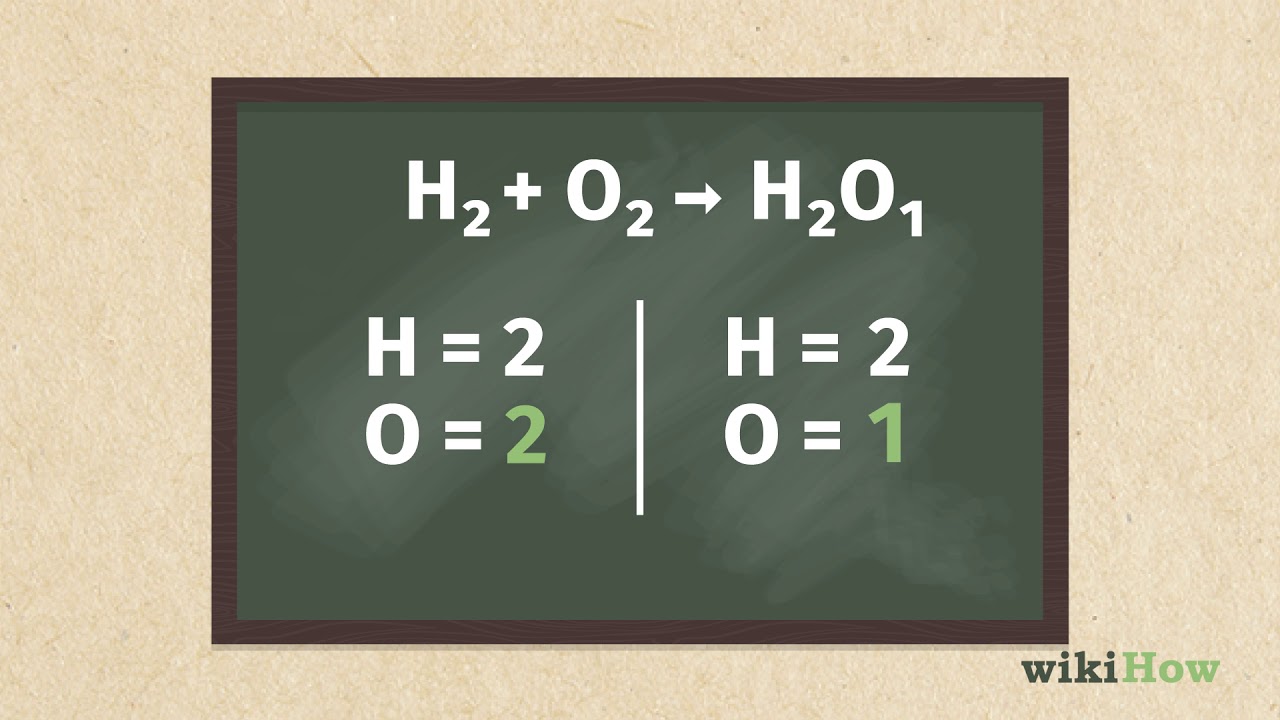
Coefficients are numbers that are placed in front of the formulas of reactants or products to balance the equation. When using coefficients, make sure to multiply the entire formula by the coefficient. For example, if you want to balance the equation 2H₂ + O₂ → H₂O, you would add a coefficient of 2 in front of the oxygen on the reactant side.
Example: 2H₂ + O₂ → H₂O
Unbalanced: 2H₂ + O₂ → H₂O (oxygen is not balanced)
Balanced: 2H₂ + 2O₂ → 2H₂O
Method 4: Checking the Charges
When balancing equations involving ions, make sure to check the charges on both sides of the equation. The total charge on the reactant side must equal the total charge on the product side. If the charges are not equal, adjust the coefficients accordingly.
Example: Na+ + Cl- → NaCl
Unbalanced: Na+ + Cl- → NaCl (charges are not equal)
Balanced: Na+ + Cl- → NaCl ( charges are equal)
Method 5: Practicing, Practicing, Practicing
The final method to master balance equations is to practice, practice, practice! The more you practice balancing equations, the more comfortable you will become with the process. Start with simple equations and gradually move on to more complex ones.
📝 Note: Balancing equations is a skill that takes time and practice to develop. Be patient and persistent, and you will become proficient in balancing equations.
What is the purpose of balancing equations in chemistry?
+The purpose of balancing equations in chemistry is to ensure that the number of atoms for each element is the same on both the reactant and product sides of the equation.
How do I know if an equation is balanced?
+An equation is balanced if the number of atoms for each element is the same on both the reactant and product sides. You can check this by counting the atoms and using the methods outlined in this blog post.
Can I use coefficients to balance an equation?
+Yes, coefficients can be used to balance an equation. A coefficient is a number that is placed in front of the formula of a reactant or product to balance the equation.
Mastering balance equations in chemistry takes time and practice, but with the five methods outlined in this blog post, you will become proficient in balancing equations. Remember to count the atoms, balance elements one by one, use coefficients, check the charges, and practice, practice, practice!
Related Terms:
- Balancing Equations Chemistry Worksheet
- Balancing chemical equations