8 Essential Facts About Atoms, Ions, and Isotopes
Understanding the Building Blocks of Matter
Atoms, ions, and isotopes are the fundamental constituents of matter, and understanding their properties and behaviors is crucial for advancing various fields of science and technology. In this article, we will delve into the world of atoms, ions, and isotopes, exploring their definitions, structures, and significance.
What are Atoms?
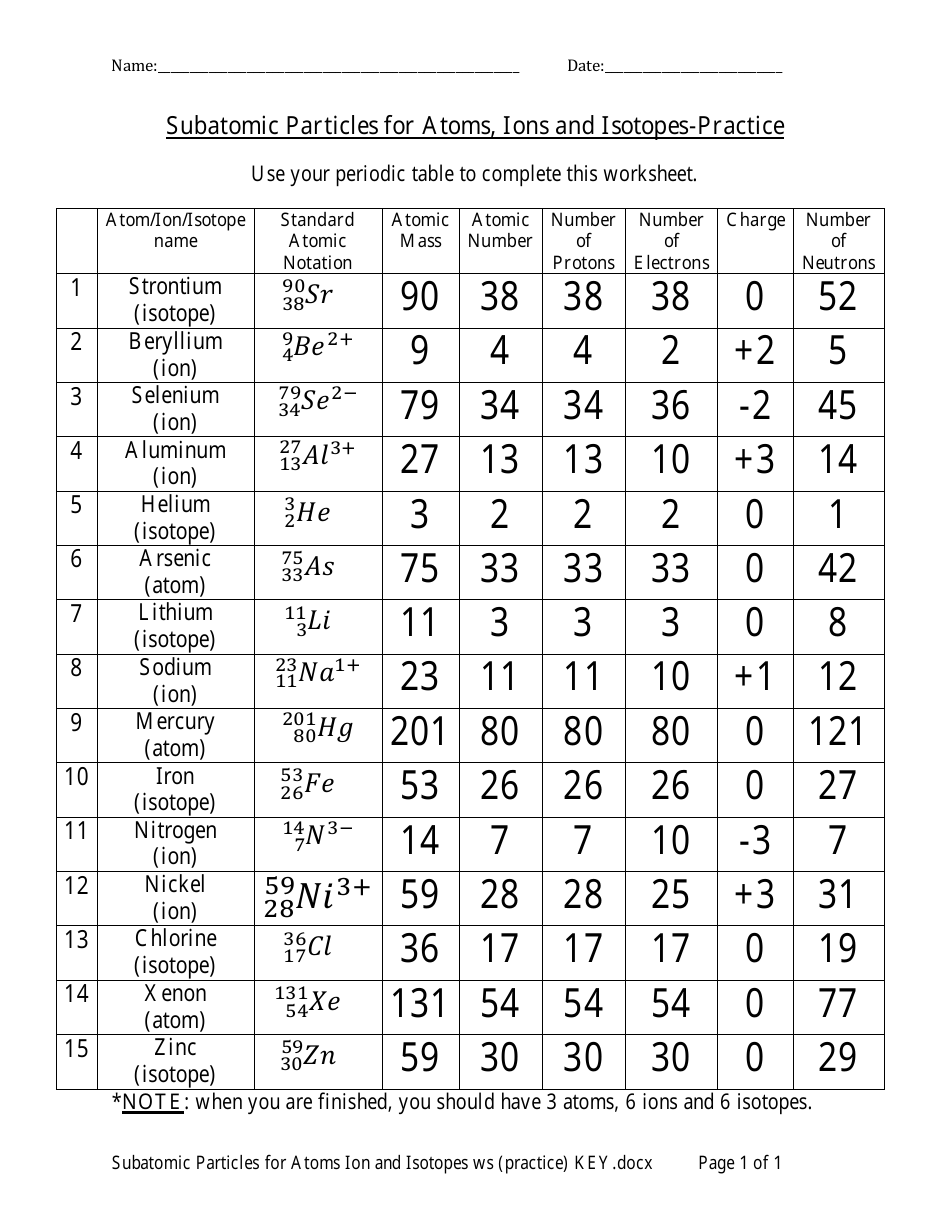
Atoms are the smallest units of a chemical element that retain the properties of that element. An atom consists of three main parts: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, which is the central part of the atom, while electrons orbit around the nucleus. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms.
Atomic Structure
The atomic structure can be visualized as a tiny solar system, with the nucleus as the sun and the electrons as the planets. The protons and neutrons in the nucleus are collectively known as nucleons. The number of nucleons in an atom is known as the mass number, while the number of protons is known as the atomic number.

| Particle | Charge | Location |
|---|---|---|
| Proton | Positive | Nucleus |
| Neutron | Neutral | Nucleus |
| Electron | Negative | Orbiting the nucleus |
What are Ions?
Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. When an atom gains or loses electrons, it becomes an ion. Ions can be either positive (cation) or negative (anion), depending on the number of electrons gained or lost.
Types of Ions
There are two main types of ions:
- Cations: Formed when an atom loses one or more electrons, resulting in a net positive charge.
- Anions: Formed when an atom gains one or more electrons, resulting in a net negative charge.
What are Isotopes?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means that isotopes have the same atomic number but different mass numbers. Isotopes can be stable or radioactive, and they can be used in various applications, including medicine, geology, and environmental science.
Types of Isotopes
There are two main types of isotopes:
- Stable isotopes: Do not undergo radioactive decay.
- Radioactive isotopes: Undergo radioactive decay, emitting radiation to become more stable.
💡 Note: Isotopes are used in various applications, including medical imaging, dating rocks, and tracing the movement of water in the environment.
Importance of Atoms, Ions, and Isotopes
Understanding atoms, ions, and isotopes is crucial for advancing various fields of science and technology, including:
- Chemistry: Atoms and ions are the building blocks of molecules, and understanding their properties and behaviors is essential for understanding chemical reactions and processes.
- Physics: Isotopes are used in various applications, including medical imaging, dating rocks, and tracing the movement of water in the environment.
- Biology: Atoms and ions play a crucial role in biological processes, including photosynthesis, respiration, and the transmission of nerve impulses.
Real-World Applications
Atoms, ions, and isotopes have numerous real-world applications, including:
- Medical Imaging: Radioactive isotopes are used in medical imaging techniques, such as positron emission tomography (PET) scans.
- Dating Rocks: Radioactive isotopes are used to date rocks and determine their age.
- Environmental Science: Isotopes are used to trace the movement of water in the environment and to study the behavior of pollutants.
What is the difference between an atom and an ion?
+An atom is a neutral particle, while an ion is an atom or molecule that has gained or lost electrons, resulting in a net positive or negative charge.
What are isotopes used for?
+Isotopes are used in various applications, including medical imaging, dating rocks, and tracing the movement of water in the environment.
What is the difference between a stable isotope and a radioactive isotope?
+A stable isotope does not undergo radioactive decay, while a radioactive isotope undergoes radioactive decay, emitting radiation to become more stable.
In conclusion, atoms, ions, and isotopes are the fundamental constituents of matter, and understanding their properties and behaviors is crucial for advancing various fields of science and technology. By exploring the world of atoms, ions, and isotopes, we can gain a deeper understanding of the world around us and develop new technologies and applications that improve our lives.
Related Terms:
- Isotope Practice Worksheet answers PDF
- Isotope Practice Worksheet pdf
- Ions Practice Worksheet