Chemical Reactions Worksheet Answers Made Easy

Chemical Reactions Worksheet Answers Made Easy
Studying chemical reactions can be a daunting task, especially when it comes to balancing equations and identifying reactants and products. However, with the right tools and resources, it can be made much easier. In this article, we will go over some common types of chemical reactions, how to balance equations, and provide some examples of chemical reactions worksheets with answers.
Types of Chemical Reactions
There are six main types of chemical reactions:
- Synthesis Reactions: Two or more substances combine to form a new compound.
- Decomposition Reactions: A single compound breaks down into two or more substances.
- Replacement Reactions: One element replaces another element in a compound.
- Combustion Reactions: A substance reacts with oxygen to produce heat and light.
- Neutralization Reactions: An acid and a base react to form a salt and water.
- Redox Reactions: The transfer of electrons from one substance to another.
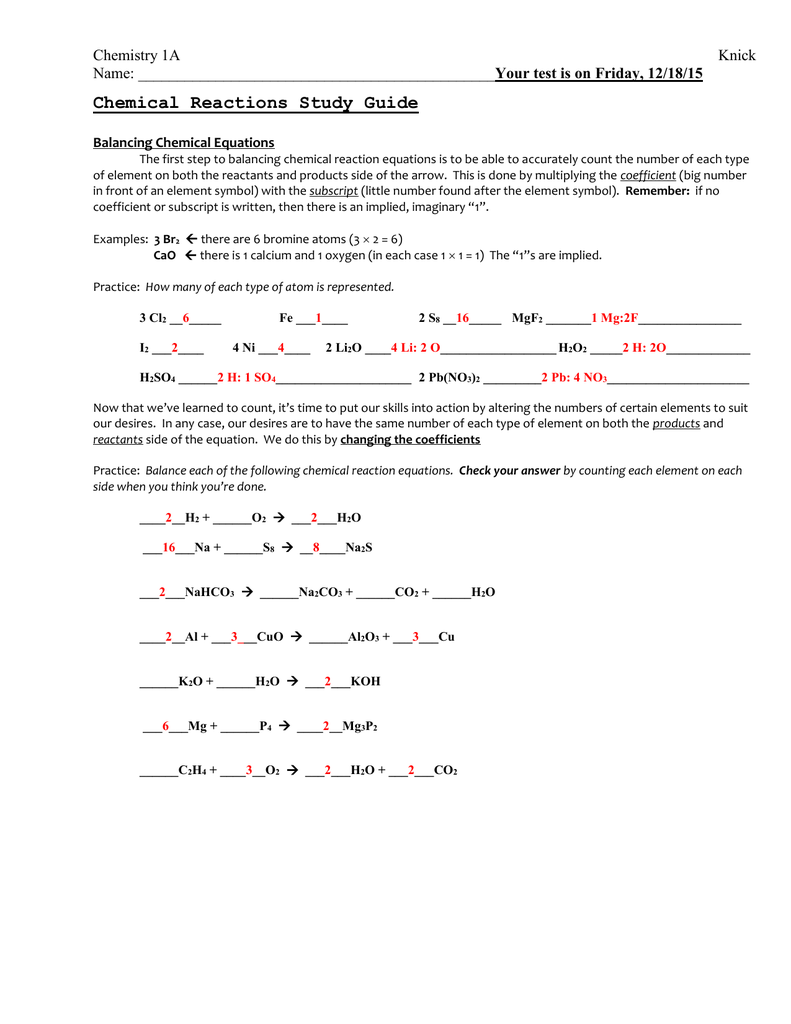
How to Balance Chemical Equations
Balancing chemical equations is a crucial step in understanding chemical reactions. Here are the steps to balance a chemical equation:
- Write down the unbalanced equation: Write the reactants on the left side and the products on the right side.
- Count the atoms: Count the number of atoms of each element on both sides of the equation.
- Add coefficients: Add numbers in front of the formulas of the reactants or products to balance the equation.
- Check the equation: Check the equation to make sure it is balanced.
Chemical Reactions Worksheet Examples
Here are some examples of chemical reactions worksheets with answers:
Example 1: Synthesis Reaction
Reactants: 2Na + Cl2 Products:?
Answer: 2NaCl
Example 2: Decomposition Reaction
Reactants: CaCO3 Products:?
Answer: CaO + CO2
Example 3: Replacement Reaction
Reactants: Zn + CuSO4 Products:?
Answer: ZnSO4 + Cu
Example 4: Combustion Reaction
Reactants: CH4 + O2 Products:?
Answer: CO2 + H2O
Example 5: Neutralization Reaction
Reactants: HCl + NaOH Products:?
Answer: NaCl + H2O
Chemical Reactions Worksheet Answers Key
Here is a key to the chemical reactions worksheet answers:
| Reaction Type | Reactants | Products |
|---|---|---|
| Synthesis | 2Na + Cl2 | 2NaCl |
| Decomposition | CaCO3 | CaO + CO2 |
| Replacement | Zn + CuSO4 | ZnSO4 + Cu |
| Combustion | CH4 + O2 | CO2 + H2O |
| Neutralization | HCl + NaOH | NaCl + H2O |
💡 Note: The above examples are just a few illustrations of chemical reactions. It is essential to practice more examples to get a better understanding of the concepts.
Tips for Solving Chemical Reactions Worksheet
Here are some tips for solving chemical reactions worksheets:
- Read the question carefully: Read the question carefully and identify the type of reaction.
- Write down the unbalanced equation: Write down the unbalanced equation and count the atoms.
- Add coefficients: Add coefficients to balance the equation.
- Check the equation: Check the equation to make sure it is balanced.
- Practice, practice, practice: Practice more examples to get a better understanding of the concepts.
In conclusion, solving chemical reactions worksheets can be made easy with the right tools and resources. By understanding the types of chemical reactions, how to balance equations, and practicing examples, you can become proficient in solving chemical reactions worksheets.
What are the six main types of chemical reactions?
+The six main types of chemical reactions are synthesis, decomposition, replacement, combustion, neutralization, and redox reactions.
How do you balance a chemical equation?
+To balance a chemical equation, write down the unbalanced equation, count the atoms, add coefficients, and check the equation to make sure it is balanced.
What is the difference between a synthesis reaction and a decomposition reaction?
+A synthesis reaction is when two or more substances combine to form a new compound, whereas a decomposition reaction is when a single compound breaks down into two or more substances.
Related Terms:
- 2 4 chemical reactions answers pdf
- 2.4 chemical reactions Section Review
- 2.4 chemical reactions and enzymes
- Enzymes Worksheet with answers